Our bodies are also… slightly radioactive
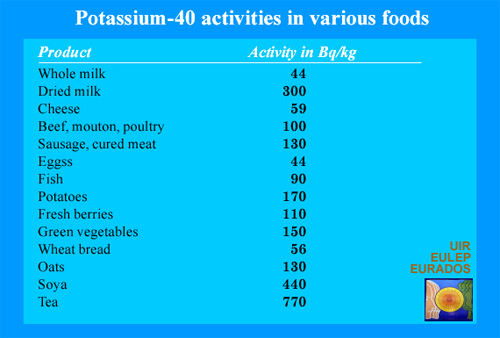
Potassium 40 in food products
Potassium atoms are present in food. It would be pointless, in order to reduce our body small potassium-40 activity, to refrain from eating soybeans or drinking tea ! Our organism needs potassium. Metabolism regulates its assimilation and that of potassium-40. The assimilation of radioactive carbon-14 present in the air, plants and living things is similar. Remembering that a becquerel (Bq) is an extremely small unit, activities of a few hundred becquerels per kilogram pose no real risk.
© IN2P3
All through our lives, we inhale and ingest radioactive elements that are naturally present in the Earth crust or produced by cosmic radiation. These elements then start irradiating our bodies from the inside.
More than half of internal radiations comes from potassium 40, which enters the human body by food ingesting it. Along with the radioactive carbon 14 isotope , eight thousand atoms decay in our bodies every second: human beings are radioactive creatures !
Atoms of potassium 40 and carbon 14 are emitters of beta electrons, particles which are immediately absorbed by the body. 11% of the decay products of a potassium 40 atom take the form of gamma rays, particles which are penetrative enough to be detected outside the body.
The primordial radioisotopes which exist (alongside their descendants) within rocks on Earth can also be found in trace quantities in drinking water, vegetation and food. These can then result in internal irradiation, in conjunction with the radioactive dust inhaled with the air.
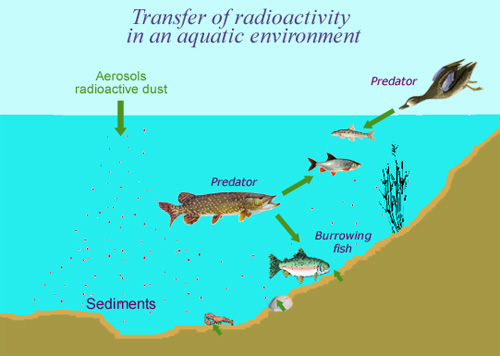
Concentration in the food chain
The example above shows how natural radioactivity can enter the food chain to affect human beings. Gaseous radon will transform into lead 210 after a few days, atoms of which will then rain down onto the surface of the Earth after being fixed on dust. The atoms found in water or in natural sediments will have plenty of time (their half-lives are of 22.3 years) to enter into the bodies of living organisms. However, the concentrations of these heavy natural radioisotopes that build up in the body are harmless.
© IN2P3
Unfortunately, these regulation processes do not exist for radionuclei originating from uranium or thorium, which means that the doses we feel are directly related to the quantities we absorb.
Of all the heavy elements which are formed by the decay of uranium or thorium, the ingestion or inhalation of lead 210 is responsible for the most important internal exposure. Uranium 238, along with its two descendants thorium 234 and protactinium 234, and uranium 234 are primarily ingested and form high concentrations around the kidneys and on the bones. Thorium 230 and thorium 232 attach themselves to the lungs, and their descendants (radium 226 and radium 228) can often be found in food.
The presence of these natural radioisotopes in our bodies on average makes up an annual dose of 0.25 mSv per year.
Other articles on the subject « Natural Radioactivity »
Natural Origins
From vestiges of earth formation to cosmic rays Despite having evolved under constant exposure to[...]
Uranium and thorium origins
Radioactive substances older than the Earth The principal source of natural radiations on Earth i[...]
Cosmogenic Radioelements
Formation of radioactive atoms from cosmic rays The Earth is constantly being bombarded by &lsquo[...]
Natural Exposure
A chronic but benign exposure All exposure to radioactivity, whether natural or artificial, is me[...]
Ground Radioactivity
Telluric Exposure : Radiation which emanates from rocks The Earth crust contains a number of radi[...]
Radioactivity in food
In our glasses and on our plates … The very acts of breathing and walking around make it im[...]