The Sun: an almost inexhaustible source of energy
Why does the Sun shine? Why has it been shining for so long?
Our Sun came into being about 4.5 billion years ago as a giant cloud of stellar matter largely comprised of hydrogen: the universe smallest and most common atom, whose nucleus is made of one single proton. This large stellar cloud was gradually compressed by gravity, and reached such high temperatures that large-scale nuclear reactions took place, producing immense amounts of energy. It is the energy released in such nuclear reactions that powers the Sun, and which is released in the form of light and heat.
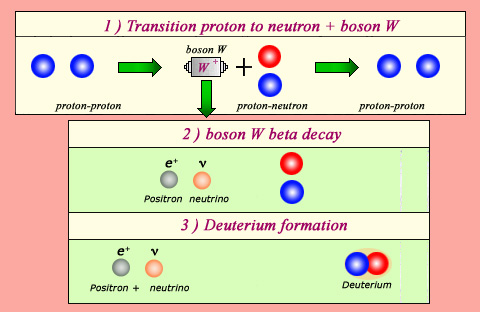
The source of fusion energy in the Sun:
The Sun gets its energy from the fusion reaction that transforms two protons into a nucleus of deuterium, a positron and a neutrino. This reaction liberates 2 MeV, and forms the neutrons which will later be used in heavier nuclei. Such processes would be impossible without the phenomenon responsible for the beta decay: the weak interaction. Weak interaction allows a proton to transform during a very short period of time into a W boson and a neutron (1). If, during these short lapses of time, this W boson decays into a neutrino and a positron (2), the neutron can bind itself with an other proton, then forming a deuterium nucleus (3).
© IN2P3
The principal reaction that takes place in the Sun uses hydrogen as a fuel. The combination of two hydrogen atoms into an atom of deuterium releases about 2 million electronvolts of energy: making these solar reactions several millions of times more energetic than the burning of petrol or coal. In order for this energy to be produced, however, the two hydrogen nuclei need to come into contact at temperatures in excess of one million degrees C.
The formation of a deuterium nucleus from two hydrogen nuclei is the Sun main energy generator, and is a fascinating reaction in its own right. Each hydrogen nucleus consists of one proton, whereas the deuterium nucleus is made up of one proton and one neutron. The process whereby a proton transforms into a neutron relies on the same fundamental phenomenon responsible for beta radioactivity: what scientists know as the ‘weak interaction’
Without the help of these weak forces, this transformation would be impossible. Two protons, even on contact, are unable to bind because they repel each other. However, each of the two protons has the ability to briefly turn into a neutron by emitting a particle called boson W. This W boson is usually immediately reabsorbed, the neutron becoming proton again. Exceptionally, it happens that the ephemeral W finds time to disintegrate into a positron and a neutrino. The neutron does not become proton anymore. It can then bind with the other proton to form a deuterium nucleus.
The formation of any star relies heavily on these ‘weak interactions’, which play a crucial role in producing neutrons. In the large stellar clouds that eventually become stars, there are virtually no neutrons to be found outside of atomic nuclei. The deuterium neutrons, therefore, will be indispensable in forming the nuclei of such essential atoms as carbon and oxygen.
Cooking over low heat…
The Sun has been shining for 4.5 billion years, and has enough hydrogen to burn for another 6 billion. It would have collapsed in on itself long ago, however, if the pressure exerted by the emitted rays did not counteract the effect of gravity.
Why has the Sun been shining for so long? The combination of circumstances leading to the fusion of hydrogen is very difficult to achieve. Hydrogen fusion occurs at a slow rate in stars. The proton-proton repulsion, and the comparative weakness of the weak interaction mean that only an infinitesimal fraction of hydrogen collisions are successful. As a result, and fortunately for us, the Sun life cycle is a very long one.