The legacy of nuclear testing and Chernobyl

Underground atomic test in French Polynesia
One of the last French underground nuclear tests around 1995. Water from the lagoon of Mururoa Atoll in French Polynesia suffered the shock wave from the basement. Unlike atmospheric tests or serious reactor accidents, underground tests do not directly release radioactive material. The radioactivity is contained in principle in depth, but contamination can occur on the surface if the rock is cracked.
© CEA/DAM
Part of the radioactivity in the environment today is due to significant releases of radioactive material occurred in the past. These releases are somehow accidental, because they are not natural but anthropological. Some are deliberate: that is the releases in air of radioactive materials due to the tests of atomic and thermonuclear bombs of the fifties and sixties. Others, involuntary, are due to real accidents : the main accidents are the Chernobyl accident in 1986 and the Fukushima one in 2011.
During this half-century atmosphere of mutual deterrence that was the Cold War, nuclear weapon, a symbol of power, was the tool of this deterrence. The great powers proceeded to many tests of atomic bombs and H bombs. At the beginning of the Cold War, the tests were carried out in air, far from inhabited places in principle. The United States have conducted many tests in the Nevada desert, then on Pacific atolls. The most famous site was that of the Bikini atoll which had been emptied of its inhabitants. France has used for its first tests in 1959-60, the firing site of Reggane in the Sahara, English used the Australian desert, and Chinese the Gobi desert. These atmospheric tests stopped in 1981. Treaties now ban them.
Atmospheric tests released radioactive dust carried by winds that have spread across the globe. They have given rise to a population exposure and contamination of the food chain by some radioisotopes. However, given their radioactive period, most of them have completely disappeared. Remain essentially caesium-137 (period 30 years), strontium-90 (28.6 years) and, to a lesser degree, krypton-85 (a noble gas of period 10.7 years) and tritium (12.3 years period). Traces of americium-241 are still found in small but significant. This radioelement, which was not dispersed during the Chernobyl accident, is an indication of these tests done very far and long ago.
The underground nuclear tests have been cleaner, fission products being in principle confined underground. But there is a risk that by cracking and the action of water, some rise to the surface. France conducted its last underground test in 1995 on the atoll of Mururoa. Nuclear tests were conducted again in 1999 by India and Pakistan.
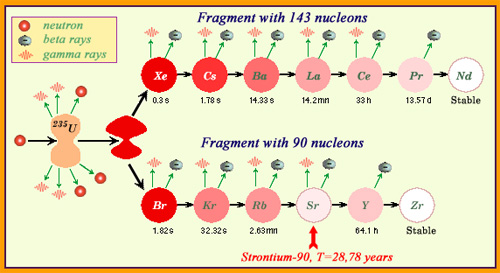
The radioactivity of fission products…
Radioactive materials released by an atomic explosion differ from those of a reactor accident. During a nuclear explosion, fission has just occurred: fresh fragments are released. These fragments are extremely radioactive due to very short half-lives, such as these of the fragment cascade at 143 nucleons of the figure. During a reactor accident, most of fission products are several months or years old. The radioelements left over come from cascades containing a long-lived radionuclide such as strontiun-90 with a 29 year half-life. The presence of short lived radioisotopes like iodine-131, with a period of eight days, comes from recent fissions.
© IN2P3
A disaster like the explosion of the Chernobyl nuclear power plant in 1986 released a large quantity of radioactive materials near a big city. The nature of the Chernobyl fallout is similar to that of an atmospheric nuclear test. Ukrainian sand Belarusians were by far the most exposed. For the population, the most serious exposure was due to a short lived iodine isotope, iodine-131.
For a country like France, the effects have been mitigated by distance and time. However, experience shows that various mechanisms can help to concentrate radioactivity. Such a concentration was observed in France after the accident in the mountains of Mercantour and the Ecrins in Southern Alps.
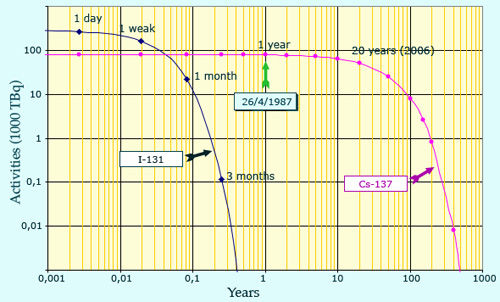
Disparition of 2 main fission products : iodine-131 and Caesium-137
This graph shows the evolution of the activity of the two main radioactive atoms dispersed during the Chernobyl accident, iodine-131 and caesium-137. One sees the effect of their very different periods (half-lives). Because of its short 8 days half-life, iodine has been very active and has prevailed in the early days, but disappeared after 3 months. Caesium was less active because of its 30 years half-life but has declined only of 50% 30 years after the accident. (Note: logarithmic scales are required to represent the very large variations of activity and the large time scale).
© IN2P3
The Alps have been more contaminated because they stopped the cloud, which stood at an altitude of 1500-3000 meters. Rain then washed the atmosphere to the detriment of the ground. Cesium atoms, accumulated downwards as a result of streaming waters. Moreover, the snow loaded with dust, melting in the valleys has contributed to local accumulations of radioactivity. It has been observed in 1998 tasks of very localized activity, up to 100 000 becquerels per kilogram. In terms of health risks, dangers remain low. It should stay about three months at such places to reach a dose of 1 mSv per year, but it is very difficult to judge from recent analysis, what was the level of pollution in 1986, when the accident, in particular that of the pollution from short-lived elements such as iodine-131, have disappeared.
Articles on the subject « Accidental Releases »
Atomic bombs legacy
A legacy of the cold war The residue of radioactive contamination which still endures has its ori[...]
Atmospheric Releases
Atmospheric radioactive pollution after a major accident During a major accident, radioactive mat[...]
Risks of ground deposits
Two deposition modes: dry deposits and wet deposits In ordinary life, apart from the vicinity of [...]
Risks of Internal Exposure
After an accident : what impact on food and man ? A large amount of radioactivity was dispersed i[...]
Exposition modes (accident)
What are the exposition pathways after a major accident ? What is the impact on man of a major re[...]