A natural radioactive gas
Radon exposure
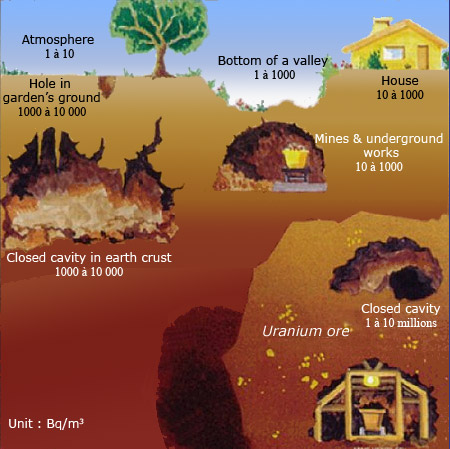
Exposure to radiations emitted by radon and its descendants is expressed in becquerels per cubic meter (Bq/m3). The risk varies mainly with conditions of ventilation. Radon is a heavy gas that has the tendency to accumulate at the bottom of valleys, in holes and caves. Released by rocks containing uranium, radon escapes from granite and volcanic rocks. It can especially be found inside uranium mines.
© DR
Radon 222 is the mais source of natural radioactivity to which humans are exposed.
This isotope of radon belongs to the radioactive lineage of uranium 238, and is its sixth generation descendant. It is formed by the decay of its precursor, radium-226. It decays within a few days into polonium 218 through the emission of an alpha particle. Its very short half-life (3.8 days) means that we would not be able to see it in our environment if it was not constantly being regenerated. The decay chain of uranium 238 is such that at any given moment as much radon is being created as is disappearing. This is the law of radioactive equilibrium.
Radon is the main source of exposure to natural radioactivity for humans because it is the only descendant of uranium to exist in a gaseous state. This allows it to leave the surface of a rock containing some uranium and enter the atmosphere. Its ability to escape from underground volcanic and granite-based rocks and enter the air that surrounds us has made radon exposure a ubiquitous feature of our terrestrial environment.
A few tens of grams of radon on the Earth surface are all that is needed to make it the most significant contributor to natural radioactivity levels. This astonishingly small quantity is the consequence of radon-222 half-life, which is very short by comparison to that of radium and even more when compared to that of uranium 238. According to the law of radioactive equilibrium, radioisotopes in a radioactive sery have an abundance inversely proportional to their half-lives: one atom for 154,000 radium atoms and one for 430 billion uranium 238 atoms.

Radonator
This curious apparatus, called radonator, was supposed in the years 1920-1930 to allow radon to circulate in bath water and provide the young woman with the supposed benefits of radiation. At the time of the photograph, the radium from which radon can be extracted was so rare and so expensive that fake powders and balms were often used instead. At the time, radon was highly valued for its radioactive properties.
Musée Curie
In a country like France, radon is responsible for 34% of the total radiation exposure felt by the population. These levels vary greatly from one place to another, with uranium-rich granite soil releasing more radon than sedimentary rocks.
Radon is an inert gas which as a result does not readily undergo chemical reactions: it can dissolve in water but does not combine with other atoms to form molecules.
The risk radon poses is mainly due to its highly radioactive descendants. Inhaled alongside radon, these solid radioisotopes emit radiation which can severely affect sensitive cells, such as those in the lungs.
A good ventilation system significantly reduces the lprobability of high concentrations of radon. Over the last few years, regulations about building construction work have been put in place to limit the possible risks of exposure.
Articles on the subject « Radon »
Radon Properties
Radon and its descendants First discovered in 1899, radon is a colourless and odourless naturally[...]
Risks of Radon
Radon: a carcinogenic agent Radon is a radioactive gas that is found in the atmosphere in minute [...]
Radon in buildings
Radon may concentrate in homes and buildings The radon which makes its way up through the soil wi[...]