A penetrative radiation more difficult to absorb
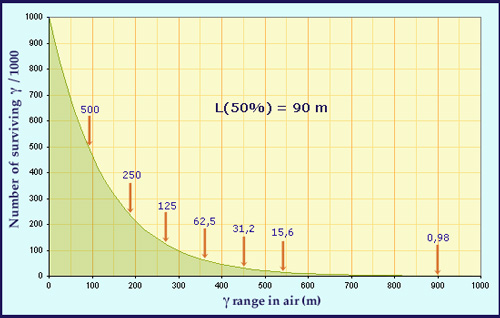
Exponential attenuation of gamma through matter
The graph above shows, for 1000 initial 1 MeV gamma rays; the number of survivihng gamma which have not interacted, as the function the distance they travel trough air. Every 90m (a distance known as the attenuation length), the number of surviving gamma rays is halved. After 2 attenuation lengths the number drops to 250, after 3 lengths to 125 and so on. This exponential decay closely resembles the way a substance’s radioactivity decreases with time. The figure of 90m is specific to 1 MeV gamma rays – less energetic gamma rays will have shorter attenuation lengths.
© IN2P3
In the case of ingestion of radioactive substances, gamma rays are far less dangerous than alpha or beta rays. As gamma photons are much more penetrative than X rays most will pass straight through the body without interacting with it at all.
It is the gamma rays emitted by an external source of radiation which pose the greatest threat. The most common radioprotection techniques with such sources consist in shielding them or interposing screens between them and the object. Obviously, one can also reduce the exposure by going away. Physicits call this the ‘solid angle effect’ .
The most effective protective shields or screens are those made of heavy atoms whose nuclei have high electrical charge. This is why most protective screens are made of lead – a heavy material (with 208 nucleons per atom) which can also be purchased relatively cheaply.
The law under which radiation intensity decreases with distance travelled in matter is similar to the way an isotope activity decreases with time : both are examples of exponential decay. The defining characteristic of such decay law is the ‘attenuation length’, which is the distance over which half the initial gamma rays have survived without interacting. Although in theory there will never be a screen sufficiently thick to ensure that all gamma rays have interacted, ten attenuation lengths are enough to ensure all only 0.1% of the original radiation survives.
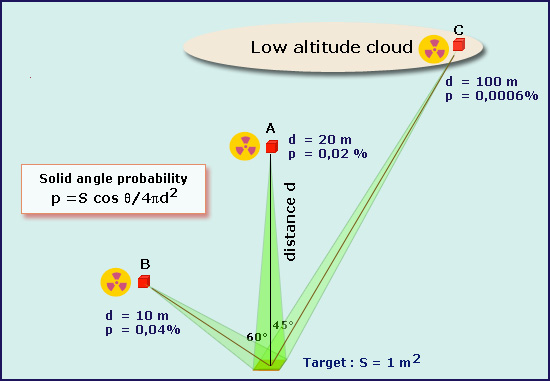
Solid angle effect
When a source emits gamma in all directions, the probability that a gamma hits a small target decreases rapidly with the source-target distance (d). The portion of space, called solid angle, which sees the target from the source decreases as the square of the distance. For distances(d) of a few tens of meters shown in the figure (A, B, C) and an area of 1 m2, the probabilities (p) that the gammahit the target are very small.
© IN2P3
Attenuation and absorption
Attenuation is not the same as absorption. The radiation which interacts does not completely disappear, as it gives rise to a secondary radiation through processes known as the Photoelectric and Compton Effects. The phenomenon is a complex one.
For radioprotection the rate of energy absorbed is more significant than the attenuation law. If the screen is sufficiently thick, however, then most of the secondary radiation can also be absorbed.
This explains why it is possible to observe safely an intense source of cobalt 60 through 20 cm of lead glass, or to look at spent nuclear fuel assemblies under 3m of water in a reactor pool.
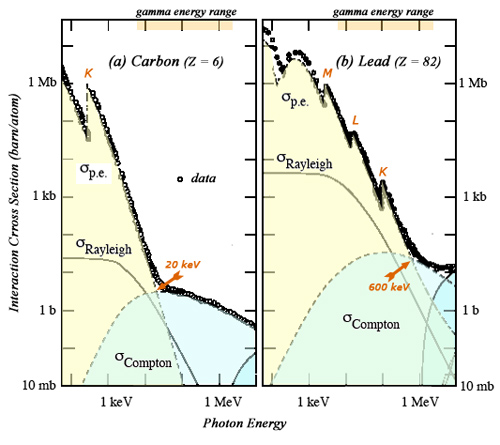
Competition between Compton and photoelectric effects
Two main processes of gamma interaction with matter compete in the energy range of radioactivity gamma (from a few keV to 1 or 2 MeV). As can be seen on the examples of carbon and lead, the outcome of the competition depends on the gamma energy, the electron density and the atomic nucleus electric charge Z. The interaction probabilities for photoelectric effect are far higher than the Compton effect in the low energy range. Compton Effect becomes dominant for energies above 600 keV for lead and only 20 keV for carbon.
© IN2P3 (Source Particle Data Group)
The gamma ray high ability to penetrate matter implies a reduced radiotoxicity. As they pass through a medium, gamma rays do not directly ionise the atoms but only transfer some of their energy to electrons with which they collide. They deposit their energy by proxy ! The secondary electrons, made to move, result in an indirect ionisation. As a result, the effects of gamma rays are not as localised as those of alpha and beta radiation, which leave a clear ionisation trail in their wake.
A significant fraction of gamma rays will leave the body without interacting : they cause no prejudice and can be picked up by external detectors. This is the reason behind the common use of gamma radiation in medical scanning techniques such as scintigraphy. The radioactive isotopes are placed inside the body, but the gamma radiation these atoms emit result in minimal irradiation of the surrounding tissue. Most scintigraphies uses technetium-99m, because this radioisotope emits only gamma rays.
NEXT : Gamma atténuation
NEXT : Gamma absorption
Other articles on the subject « Radioprotection »
Radioprotection principles
Applying common sense rules according to radiation nature The need for protection from radiation [...]
Gamma Attenuation
Attenuation of a gamma beam Of the three types of radiations, gamma rays are the most penetrative[...]
Gamma Absorption
Heavy materials for the absorption of gamma rays In terms of radioprotection mitigate gamma rays [...]
Radioprotection neutrons
A rare radiation, dangerous, penetrating, difficult to absorb The neutron radiation is more penet[...]
Radioactive Decontamination
Avoid all contact, ingestion and inhalation One of the tasks of radiation protection is the decon[...]
Justification and optimisation
In medicine, use of radiation must be justified The ‘Justification and Optimization » princ[...]
Reglementations and controls
Strict regulations for an efficient radioprotection Radioprotection has a long history. Its princ[...]
Dose legal limits
Applying the precautionary principle and setting cautious limits French regulations set at 1 mSv [...]
Actors in radioprotection
The actors and the basics of radioprotection regulations The rules of radiation protection are no[...]