The most effective mechanism of photon absorption
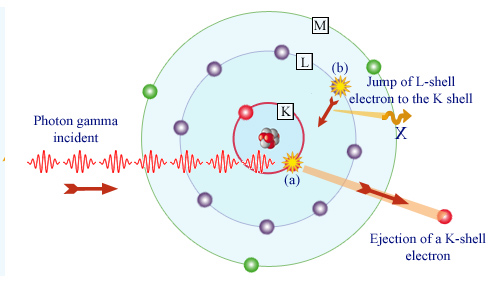
Gamma absorption by an atom
The photoelectric effect occurs in two steps. First, the photon (a) takes out a bound electron in one atom. In the case of gamma photons, it is usually an electron belonging to the innermost layers L or K (as shown). The photon is absorbed and the atom that has lost one of its inner electrons is left in an excited state. An electron from an outer layer (b) moves to occupy the vacancy left by the ejected electron. If the ejected electron belonged to the K-shell as in the figure, an X-ray is emitted during this transition.
© IN2P3
The photoelectric effect is the phenomenon that transforms visible light, infrared and ultraviolet rays into electricity in solar panels and cells of our cameras. It is also involved in the completely different field of radioprotection : by transforming penetrating X and gamma rays into electrons easy to stop, it protects us from the effects of these radiations.
The photoelectric effect is the most effective physical phenomenon in mitigating these radiations. The gamma or X photon, absorbed by interacting with an electron bound to an atom disappears.
The shell structure of atoms plays a crucial role. The photon wrest an electron only if its energy exceeds the binding energy of the electron on its shell. The probability (called cross section) to remove an electron from this shell becomes non-zero beyond this threshold.
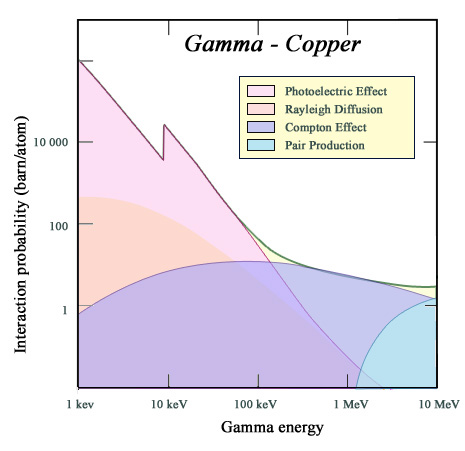
Photoelectric versus Compton effects
The curve represents, in the case of copper, the probability of gamma interaction (or cross section) as a function of their energy. The gamma stopping power is measured by this quantity. The figure shows the contribution of various mechanisms of interaction, the main ones in the energy range of radioactivity beeing the photoelectric and Compton effects. For an absorber such as copper, the photoelectric effect is much weaker than for lead (the threshold of the K-shell is less than 10 keV and nuclear charge of 28 instead of 82). The Compton effect prevails above 120 keV.
© IN2P3
The photons of visible light whose energies are small can only eject the least bound electrons of the atom which are the most external. This property rapidly decreases, until the photon energy exceeds the binding energy of the atom first inner shell : the photon becomes able to extract electrons from this shell.
The probability of pulling out electrons from the new layer decreases in turn until the photon energy exceeds the binding energy of electrons from the second inner shell, which then become the main contributors. As and when its energy increases, the photon interacts in turn with deeper and deeper atom layers.
The two deepest K-shell electrons, the closest to the nucleus, constitute somehow the ultimate cartridge of the process . After a last jump, the cross section decreases inexorably. Meanwhile, the light photon has become an X or gamma ray. In this energy range, the electric charge Z of the nucleus occurs at the fourth power: the photoelectric effect for a lead atom (Z = 82) will therefor be 10,000 times stronger than for oxygen (Z = 8 ).
The decrease of the photoelectric effect with energy is impressive, although the fall is mitigated by the jumps due to the crossing of thresholds of the successive atomic shells.
In the case of light atoms like oxygen, the binding energy of the K-shell, is of the order of 1 keV. It is negligible for gamma having energies of tens or hundreds of keV. The electron them seem almost free. We are in the Compton effect range that prevails then on the photoelectric effect.
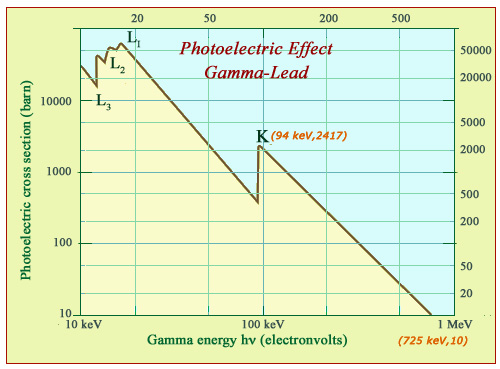
Photoelectric effect: the example of lead
In the case of lead atoms, the probability of the photoelectric effect decreases by several orders of magnitude with the energy of gamma rays. The lead photoelectric stopping power decreases by more than 2000 times in the energy range shown. This impressive decrease is however hampered by the crossing of interaction thresholds of the photon with electrons of the atom’s innermost layers L and K. For a lighter absorber such as copper, the curve would be similar but shifted left and downwards.
© IN2P3 (Source Encyclopaedia Britannica)
Heavy atoms, such as lead, areis much more favorable for the photoelectric effect and radioprotection. The material is very dense. The binding energies of 20 and 90 keV of L and K inner shells are much larger. Gamma absorption benefits from the contributions of L and K electrons and especially from the very high electric harge of the lead nucleus (Z = 82) in an area that encompasses all X-rays and a significant proportion of gamma rays.
Desexcitation X-rays : Finally, what happens to the atom left in an excited state? It inherits a surplus of energy equal to the binding energy of the expelled electron. The atom will reorganize itself and return this surplus. If the gamma has removed a K-shell electron, an electron belonging to the higher L shell will fill the vacancy left on the K-shell. During the transition a characteristic X-ray is emitted. This emission of desexcitation X-rays is sometimes called X fluorescence. When the X-ray is emited in dense matter it is usually absorbed after a short range.
NEXT : Compton Effect
Other articles on the subject « Radiations effects in matter »
Charged Particle Effects
A gradual loss and transfer of energy Alpha rays, fission products ; heavy, slow and ionizing par[...]
Alpha Rays in Matter
An atomic bulldozer, strongly ionizing along a very short path Alpha particles are simultaneously[...]
Beta Rays in Matter
Light electrons : a chaotic journey through matter Beta electrons and positrons have equal and op[...]
Bremsstrahlung
A relativistic phenomenon that applies to electrons and positrons… The phenomenon of bremss[...]
Neutral Particle Effects
Energy transfer by proxy… Neutral particles that are of interest in the field of radioactiv[...]
Cherenkov Effect
When an electron goes faster than light in air and water … The Cherenkov effect occurs when[...]
Cross Section
Cross section or the interaction probability of a particle Cross section is the name given by phy[...]
Gamma Rays in Matter
Gamma can be attenuated but never fully stopped The neutral gamma rays leave very different effec[...]
Compton Effect
Photons as projectiles and electrons as targets The Compton effect is the name given by physicist[...]
Macroscopic Effects
Effects on inert or organic matter The ionisation of atoms surrounding the trajectory of an alpha[...]