Effects on the living are dose dependent
The study of the way in which exposure to radiation can affect living matter lies at the boundary of physics and biology. Even with recent advances in scientific understanding, our grasp of this field remains incomplete and empirical. To predict the effect of radiation on a person would require at least the knowledge of the exact doses of radioactivity to which each of his organs is subjected. This information is rarely available, even more when the effect occurs years after an exposure, which is the general case. It is then impossible to link the effect with this bygone exposure since the effect may often be attributed to many other causes. When it comes to cancer, unfortunately, such definitive links can almost never be made.
Living matter is sensitive to radiation
Living cells are sensitive to all chemical or radioactive attacks. Despite the comparative fragility of DNA molecules, they have the remarkable ability to repair themselves if the damage done is not too great.
© CEA
Radiation can be beneficial to a living organism if it affects only sick or harmful cells. When it starts to touch healthy cells, however, it can have harmful consequences. Fortunately for us, most living matter has the ability to regenerate after being exposed to weak doses of radiation. For stronger doses, however, the effects are almost always irreversible.
A huge range of factors are involved when it comes to determining how harmful exposure can be. The nature of the radiation, the amount absorbed and the part of the body affected are all important in determining the eventual consequences.
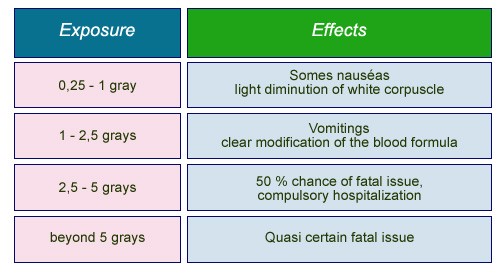
Effects of high exposures
‘Deterministic’ effects linked to a strong, homogeneous irradiation over the whole body.
© IN2P3
In the case of heavy doses (which can be beneficial in localised applications: as is common in radiation therapy) the effects can be clearly defined, and are referred to as deterministic.
These consequences become less clear when discussing doses of the same strength as naturally-occurring radiation. Such exposure is almost always harmless but can, in rare cases, lead to problems such as cancer. The low probability associated with such negative outcomes has led to them being referred to as probabilistic.
When discussing a large exposed population, it would be impossible to attribute the appearance of an individual cancer to any particular cause. The observation of noticeable (statistically significant) phenomenon, however, would be a good indication of causality.
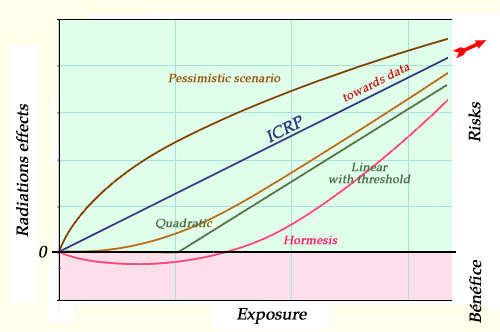
Uncertainty linked to weak doses:
In the field of the small and very small doses, the absence of precise data makes all cause-effect links uncertain. Some radioprotectionists question the validity of the ‘official’ relationship (represented by the straight line « ICRP » linking the origin to data samples at much higher doses which are outside the graph). They advocate a ‘threshold’ model – with no discernible risks below a certain threshold. A third model, known as hormesis, assumes that low doses of radiation may even be beneficial. These models are loosely sketched on the graph above, the scales on the two axes not specified.
© IN2P3
Internal radiation is the most harmful, as radioactive atoms trapped in the body can stay there for years, working over very small distances. Such an exposure is much more dangerous than external radiation, which stops as soon as one is no longer exposed to the source.
The comparative risk posed by radioactive substances is measured through their potential radiotoxicity – the dose that would be felt if the sample were to become assimilated by a human being. For the same amount of ingested radioactive sample, heavy alpha ray emitters are some 10,000 times more radiotoxic than the lightweight beta emitters such as tritium.
It is important, however, not to confuse radiotoxicity with the real danger posed by a substance. The danger posed by a radiotoxic sample is real only if one were to swallow it or breathe it in. As long as radioactive elements remain outside our bodies, radiotoxicity does not provide relevant information as to comparative danger. For instance, a very high radiotoxicity nuclei such as plutonium, is a comparatively low-risk element due to its unlikelihood of ever coming in contact with live beeings.
Articles on the subject « Radiation Effects »
Deterministic effects
The domain of strong doses and severe, reproducible effects For high doses above a certain thresh[...]
Dose-effects Relationship
Can effects caused by low doses of radiations be predicted ? Even though we are constantly expose[...]
Cumulative Dose
What is the cumulative exposure? We receive an average of 2.5 millisieverts (mSv) per year from n[...]
Dose Rate
Acute and chronic exposures The « effective dose » is not sufficient by itself to characterize on[...]
Hiroshima Nagasaki Survivors
An exces of cancers and leukaemia among survivors Our knowledge of the risks of cancers due to ra[...]
Linear No-threshold Model
A Precautionary Principle applied to radioactivity … Is it possible to predict the effects [...]
Low doses effects
Do low doses of radiation have any effect? Radioprotection experts commonly refer to doses below [...]
Radiosensitivity
Sensitivity of DNA in living cells to radiation Although we still know little about the effects o[...]
Radioactive Toxicity
A useful indicator but to be used appropriately … The danger presented by a radioactive sub[...]
Dose Factors
What doses when swallowing or inhaling radioactive atoms ? How to evaluate the doses resulting fr[...]