Heavy materials for the absorption of gamma rays
In terms of radioprotection mitigate gamma rays is not enough: they have to be absorbed. When the gamma disappears by interacting, it transmit its energy to other particles that will deposit this energy in the medium if they are charged. If they are neutral, they will continue their way until they interact themselves. The way the energy is absorbed is therefore complex.
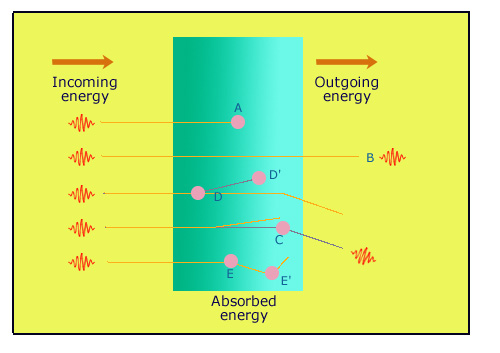
Absorption of gamma rays
Gamma rays deposit their energy inside a screen in many ways. In (A), a photon interacts by photoelectric effect: the electron is stopped almost right away. There is total absorption. In (B), the photon passes through the screen without interacting. In (C) the photon interacts through Compton effect: The Compton electron energy is absorbed locally, however the photon escapes. In the two last cases, there is total absorption because the photons are absorbed later on in the screen in D’ and E ». The absorbed energy is the difference between incoming energy and outgoing energy.
© IN2P3
The simplest case occurs when the photon interacts by photoelectric effect, that’s to say when it is absorbed in one stroke by an atom of which it ejects an electron. In a dense screen matter, the path traveled by this electron is very short so that we can consider that its energy is locally absorbed. For its part, the absorbing atom that has lost an electron will restore the energy gained by emitting one or more photons. These secondary photons, which can be X rays if the electron belonged to the innermost shell (layer) of the atom, have lost the memory of the direction of incident gamma and are emitted in all directions.
The most complicated case occurs when the photon interacts by Compton effect. As for the photoelectric effect, the photon is absorbed, but a new lower energy photon is also emitted with the ejected electron. The Compton electron as the photoelectric electron deposits its energy locally, but the new gamma escapes, taking its energy fraction. It is emitted preferably in the direction of the incident gamma. If the screen is thick, it has a better chance to be absorbed later on by interacting itself. But it can also exit the screen, in which case only a part of the energy – the electron one – has been absorbed.
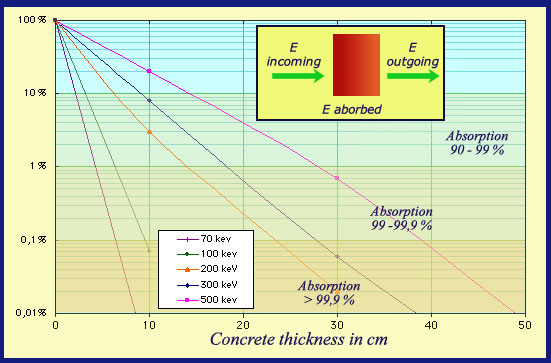
Gamma absorption in concrete
The absorption capacity of concrete is lower than that of lead, because its density is lighter (2.35 against 11.6) and this material does not contain heavy nuclei. The screens thicknesses needed amount to tens of centimeters, but concrete is cheap and screens easy to manufacture. The figure shows that a 40 cm concrete screen absorbs 99.9% of a 500 keV gamma energy. The curves are indicative because they depends on the beam characteristics.
© Source K.Gerber
These secondary gamma, which are emitted at different angles and with various energies complicate the absorption law. They not only extend the life of the beam, but they degrade it. However, it is customary to consider an absorption coefficient of the deposited energy (the electron one). The absorptive capacity ignores the deposited energy of the secondary gamma absorbed further into the screen. Concerning radiation protection, it does not matter: the screen thicknesses thus calculated overestimate the risk.
Lead is a very efficient absorber. First it is a very dense material. Then the lead nucleus is a heavy nucleus whose property is to favour the photoelectric effect. This effect plays a predominant role for gamma which, up to an energy of 200-300 keV, are mostly stopped. Lead performances are less good for more energetic gamma. The relevant figure is that only 1.5 cm screen is enough to absorb 50% of the energy of 1 MeV gamma.
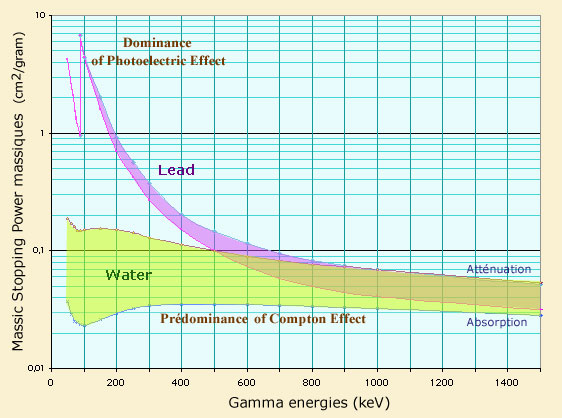
Comparison of attenuation and absorption powers in lead and water
Comparison of stopping and attenuation powers as a function of the gamma energy. Attenuation and absorption powers are rather close to each other when the photoelectric effect dominates. Lead is a very effective energy absorber up to 200 MeV, because of the dominance of the photoelectric effect for heavy nuclei. In the case of a light absorber such as water, the Compton effect dominates: the stopping power is much weaker. Variation with energy is less marked.
© IN2P3 : Sources B.Tamain et K.Gerber
Light absorbers (water, concrete, air) require greater thicknesses because they are less dense and that the photoelectric effect – beneficial to stop the gamma – plays a lesser role.
NEXT : Gamma Attenuation
Other articles on the subject « Radioprotection »
Radioprotection principles
Applying common sense rules according to radiation nature The need for protection from radiation [...]
Gamma Radioprotection
A penetrative radiation more difficult to absorb In the case of ingestion of radioactive substanc[...]
Gamma Attenuation
Attenuation of a gamma beam Of the three types of radiations, gamma rays are the most penetrative[...]
Radioprotection neutrons
A rare radiation, dangerous, penetrating, difficult to absorb The neutron radiation is more penet[...]
Radioactive Decontamination
Avoid all contact, ingestion and inhalation One of the tasks of radiation protection is the decon[...]
Justification and optimisation
In medicine, use of radiation must be justified The ‘Justification and Optimization » princ[...]
Reglementations and controls
Strict regulations for an efficient radioprotection Radioprotection has a long history. Its princ[...]
Dose legal limits
Applying the precautionary principle and setting cautious limits French regulations set at 1 mSv [...]
Actors in radioprotection
The actors and the basics of radioprotection regulations The rules of radiation protection are no[...]