Three modes of exposure : internal, by contact and external
When ionising rradiations interact with matter they are capable of expelling electrons away from their orbits in atoms. The biological effects resulting from such radiation largely depends on the dose absorbed. Exposure to radioactivity or exposure to radiations ? For the man on street as well as the patient on the hospital bed, it is only the radiations which matter – their origins are only relevant to the physicist.
Radioactivity is a common source of iradiations, but it is not the only one! Naturally, we are all constantly exposed to rays from uranium found in rocks, carbon 14 in the atmosphere, radon in the air, and the after-effects of nuclear bomb tests and Chernobyl. We also, however, feel the rain of particles from cosmic radiation hitting the Earth atmosphere. Neither these cosmic rays, nor the X-rays emitted by medical equipment, are the result of radioactive processes.
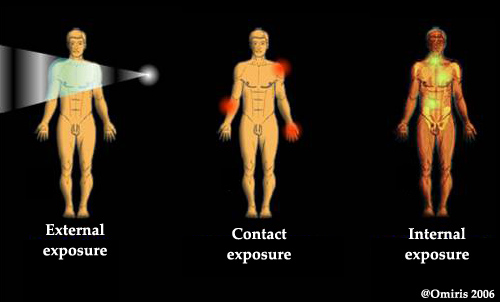
Three modes of exposition to radiation
Exposure to ionising rays, whether from a radioactive source or not, can occur internally, externally, or through direct contact. For external sources, the important factors are the duration of the exposure, the strength and distance of the source as well as the location of any potential screens. Exposure through contact is rare, and normally involves the handling of contaminated material. By far the most dangerous of the three, however, is internal irradiation, whose consequences depend on the absorption mode (ingestion or inhalation), the organs affected and how long the radioactive atoms will remain in the body.
© OMIRIS
There are two principal types of exposure to radiation : internal and external. Internal exposure occurs when the source of ionising radiations is located inside the organism, usually as a consequence of ingestion or inhalation of radioactive substances. This sort of exposure is frequently used in medicine, when radioactive tracers are placed inside the body for a diagnostic or therapeutic purpose.
External exposure, on the other hand, takes place when the radioactive source is located outside the body.
Internal exposure is the most dangerous as the radioactive atoms are fixed to your body. Alpha rays, which would ordinarily be stopped by a few cm of air or the thickness of a shirt, can now deposit their energy directly into our cells. The same is true, albeit to a lesser extent, for beta rays. These two types of radiation, due to alpha and beta particles, only affect a very localised area along their short trajectories. Gamma rays, on the other hand, are very penetrating and can even pass through the entire the body without interacting.
The low levels of naturally-occurring radiation mean that we all have a small number of radioactive atoms inside our bodies. The water cycle as well as the food chain both contain trace amounts of radioisotopes: carbon 14 is absorbed by vegetation, potassium 40 is found in the soil, and traces of uranium, thorium and their descendants can be found into some mountain water.
We also all breathe in trace quantities of radon gas – another descendant of uranium. This gas chemically inert would be harmless, were it not for the fact that its radioactive descendants may fix themselves to bronchi in the lungs. The risk associated with the inhalation of radon is the primary natural source of irradiation. In France, radon is responsible for around 55% of the effective dose of radiation due to natural radioactivity.
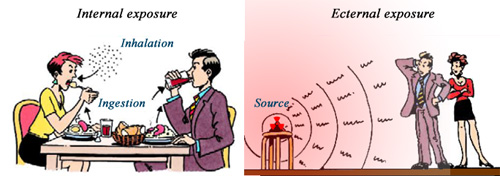
Internal and external exposures to ionising rays
Exposure to radiations comes from internal and external sources. Internal exposure results from the ingestion of radioactive atoms present in food and drink as well as the inhalation of radioactive isotopes carried by air-borne particles. If these radioactive atoms attach themselves to our organs, glands or bones, then their subsequent decay may damage our cells and affect our health. External exposure results from the exposure to the rays emitted by a radioactive source, a source of X-rays or can be natural (for example cosmic rays, radioactivity of rocks).
© IN2P3
The external exposure is the less dangerous. This time, it is the gamma rays passing through the body that are responsible of the hazards, alpha and beta particles being stopped by the outermost layers of the skin. Alpha and beta particles can be still dangerous in the case of contact with a radioactive substance and are used do destroyed malignant cells in brachytherapies.
Iodine 131, a radioactive isotope of iodine, provides an effective illustration of the different risks associated with internal and external exposure. Iodine attaches itself very easily to the thyroid – a small gland which plays a crucial role in early childhood and adolescence. After the Chernobyl accident, quantities of iodine 131 contaminated fresh products (such as milk, meat and vegetables) which were eventually ingested, leading to radioisotopes entering the thyroid. This exposure lasted for the three months following the accident. The UNSCEAR report on the health consequences of the Chernobyl accident had shown a significant increase in the risk of thyroid cancer among children and adolescents living near the reactor. This increase has been definitively linked to the quantities of iodine 131 which were released into the air.
Although gamma rays emitted by iodine-131 were also present, their effects were evenly spread throughout the body and negligible compared to effects of the iodine 131 beta rays concentrated in the thyroid.
Articles on the subject « Types of exposure »
Contact Exposure
Avoid contact with radioactive substances The direct contact of radioactive sources with skin pro[...]
External Exposures
A generally transient exposure due to gamma rays The majority of exposure to radiation is externa[...]
Internal Exposure
Radioactivity Trojan Horse… Radioactive atoms have two ways of sneaking past our body natur[...]