How nuclei get rid of excess energy
It was in 1900 that the French physicist Paul Villard first found evidence for gamma radiation. The fact that this radiation, unlike both alpha and beta rays, was not deflected by either electric or magnetic fields led to the conclusion that gamma radiation was carried by electrically neutral particles later identified as ‘photons’.
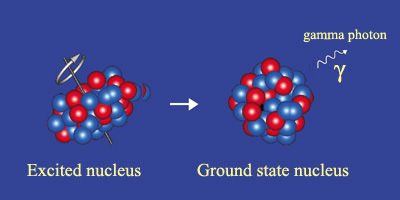
Example of gamma radioactivity
Gamma radioactivity takes place when a decay, or an event like a neutron capture, has left the nucleus with an energy surplus. The ‘excited’ nucleus generally returns to its ground state very quickly. The figure shows a deformed nucleus rotating around an axis before returning to its spherical shape and losing its rotation through the emission of a gamma ray. The gamma rays are of the same nature as photons that form the light emitted by atoms, but have energies several hundred thousands of times greater.
© IN2P3
These ‘gamma ‘ (γ) rays are of the same nature as X-rays or even the light emitted by atoms. The energy they carry away, however, is far larger; anywhere between 100,000 and a million electronvolts (MeV).
The term of gamma radioactivity is slightly misleadiong, as it is a desexcitation phenomenon similar to atomic desexcitations.
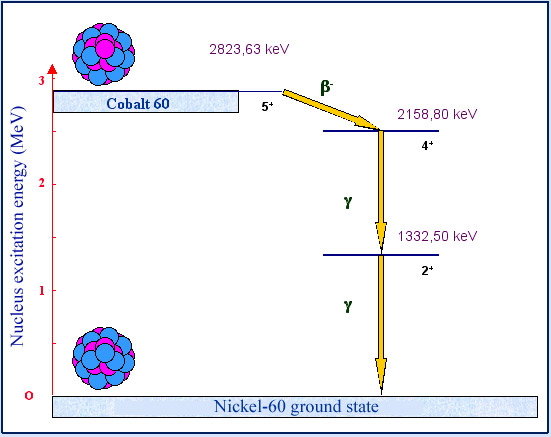
Cascade of gamma
Gamma radioactivity generally accompanies alpha or beta decay, as this example of cobalt 60 shows. The nucleus of Cobalt-60 (a radioisotope with a half-life of 5,271 years) decays by undergoing beta radioactivity and forms a stable nucleus of nickel 60. The transformation, accompanied by the emission of an electron and an antineutrino, results almost always in an excited nickel 60 nucleus. The nucleus loses the 2,158.80 keV of its excess energy by emitting a first gamma photon, followed by a second. The emission of the two photons follows that of the electron and the antineutrino.
© IN2P3
The emission of a gamma ray often follows rarely the emission of an alpha particle, but frequently a beta decay, or a neutron capture by a nucleus. These events leave the nucleus in an excited state, possessing more energy than its ‘ground’ state, which causes it to release the extra energy through the emission of one or more gamma photons, the ‘grains of electromagnetic energy’.
The gamma emission is almost always instantaneous, though it can occasionally take place with a delay. This is the case with technetium in its excited state; a state which can last for several hours and thus allows technetium to be used as a pure gamma source in hospital scans.
In an interesting parallel with the atom, nuclei have well-defined energy states. The jump from one energy level to another is accompanied by the emission of a gamma ray with a specific energy value, characteristic both of the specific energy transition and of the nucleus involved. Measuring the energy of the gamma rays emitted therefore allows for positive identification of the emitter nucleus.
NEXT : Nuclear Desexcitations
NEXT : Internal Conversion
Other articles on the subject « Alpha Beta Gamma rays »
Alpha (α) radioactivity
How heavy nuclei lose weight … by emitting alpha particles Alpha (α) radiation was first ob[...]
Beta (β) radioactivity
How Nature corrects an excess of protons or neutrons Beta (β) radioactivity was first observed in[...]
Beta spectrum
Beta electrons do not have a unique characteristic energy Three particles, the recoil nucleus, th[...]
Electron Capture
A minor mode … competing with positron emission Electron capture is a comparatively minor d[...]
Positron
The positive electron, the first ambassador of antimatter The positron was discovered in 1933 by [...]
The electron-neutrino
An electron that would have lost its electric charge The easiest way to conceive a neutrino is to[...]
Muons
An heavy electron abundant in cosmic rays Muons were first observed in 1936 by the Americans phys[...]
Nuclear Desexcitations
Gamma are the light emitted by atomic nuclei A french proverb used to say « all roads go to Rome.[...]
Internal Conversion
When a gamma expells an atomic electron and is absorbed Internal conversion is a nucleus desexcit[...]
Nuclear Transmutations
The ancient dream of the alchemists… The alchemists of the Middle Ages and the Renaissance [...]