Avoid contact with radioactive substances
The direct contact of radioactive sources with skin produces burns that can be severe if the source is intense. The effect is known since the early days of the discovery of radioactivity.
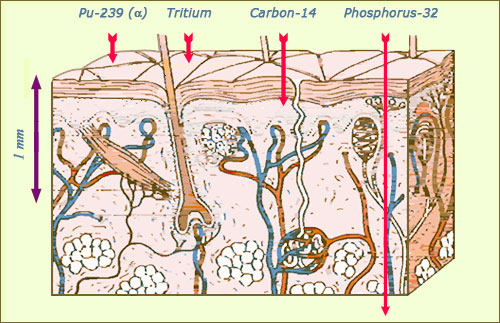
Alpha and beta trajectories within the skin
The alpha rays, like the ones emitted by plutonium, or the beta electrons of very low energy emitted by tritium have a very low penetrating power. If the radioactive source comes into contact with the skin, they are stopped in the upper layer of the epidermis. The more energetic beta electrons from carbon 14 can reach as far as the dermis, while the energetic electrons emitted by phosphorus 32 and strontium 90 can even penetrate the hypodermis.
© Source K.G.Gerber/EURADOS
In 1901, Pierre Curie put radium salt on his arm for over ten hours, in a repetition of an earlier experiment by the German chemist F. Giesel. The skin of both men became increasingly red over the following days, displaying all the usual symptoms of a burn. Wounds eventually formed which took nearly two months to heal. Pierre Curie quickly got in touch with dermatologists to explore possible therapeutic effects of such radiation – the birth of a technique known as ‘brachytherapy’.
The skin burns caused by contact with radioactive substances are due to beta rays whose energies are above 100 keV
rays whose energies are above 100 keV . Alpha
. Alpha particles emitted very close the skin are not able to penetrate deep enough to reach living tissue, as the ten or so micrometres of their path do not take them through the layer of dead cells which coats the epidermis. However it may happens, like in the case of tritium or soluble compounds, that radionucleides can be absorbed by the skin. Exposure of the skin and of deeper subcutaneous structures depends on whether or not the radiation involved is penetrative.
particles emitted very close the skin are not able to penetrate deep enough to reach living tissue, as the ten or so micrometres of their path do not take them through the layer of dead cells which coats the epidermis. However it may happens, like in the case of tritium or soluble compounds, that radionucleides can be absorbed by the skin. Exposure of the skin and of deeper subcutaneous structures depends on whether or not the radiation involved is penetrative.
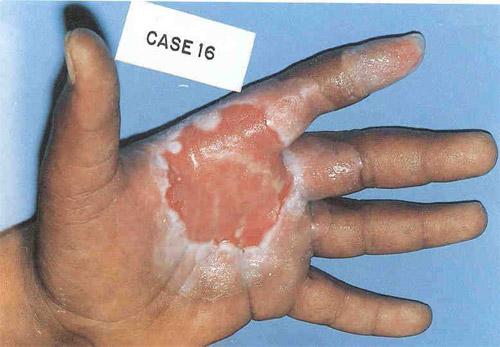
Burns caused by contact with caesium
On the 10th of September 1987, iron peddlers in the Brazilian town of Goiania picked up a small disused metallic barrel containing caesium 137, a radioactive substance used in an hospital for the treatment of cancer. The barrel was opened and small pieces of the metallic isotope, which gave off an ‘attractive blue light’ were sold on to curious passers-by. The handling of this bright powder resulted in severe burns as shown above, as well as in 129 cases of internal exposure.
© DR
The energies of beta rays can vary from a few keV to over 2 MeV. They deposit less energy than alpha particles over their trajectories, and are consequently more penetrative. When their energy is of the order of a few keV (as is the case with beta particles emitted by tritium), they can be stopped by a layer of skin. When their energies are a hundred times stronger, they can reach as far as the layers of the dermis. For energies of over 1 MeV, beta particles can reach the deepest subcutaneous layers.
In the case of a skin wound, radioisotopes can easily pass through the cutaneous layer and enter the blood. The consequences of such contact are similar to those of an internal exposure due to ingestion.
The fundamental rule of radioprotection is that radioactive substances must never come into contact with bare skin. Gloves and tongs are the minimal protection that should be employed, and even so only when contact is unavoidable. ‘Sealed radioactive sources’ are normally safe under normal conditions of use, conditioned as they are in hermetic envelopes. The level of caution must always in proportion to the intensity of the source and the possible risk of exposure.