A useful indicator but to be used appropriately …
The danger presented by a radioactive substance is often rated through an indicator called « potential radioactive toxicity » or radiotoxicity. This radiotoxicity concerns internal expositions, external expositions being considered separately. Internal expositions are the more harmful since we are not protected against alpha and beta emitters which are settled durably in our bodies. Gamma are less dangerous because depositing their energy in a diluted form while a substantial fraction may escape without interacting.
Potential radiotoxicity is an indicator mostly used in radioactive waste management to assess the potential hazard associated with ingestion. For example, a spent fuel discharged from a reactor contains kilograms of plutonium. What would be the dose resulting from the ingestion of these kilograms of plutonium by a population ? Since such an ingestion would not happen with a good waste management, the potential radiotoxicity overestimates much in this case the risk. But the indicator is useful for comparing the solutions envisaged for the very long-term storage of radioactive waste.
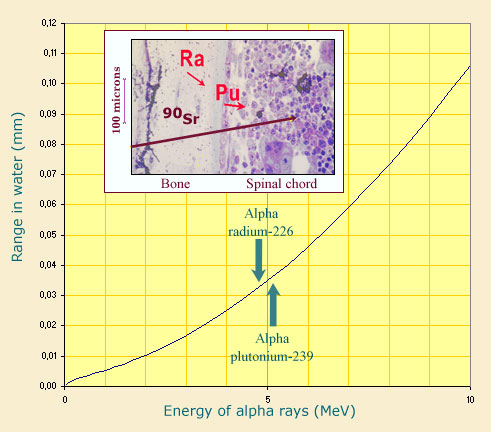
Internal exposures : alpha strong radiotoxicity
The curve illustrates the dangers of mainly alpha emitters fixed in the body where the radiation can cause the most damage. In tissue whose density is similar to water, alpha emitters such as radium and plutonium deposit their 5 MeV of energy over a short path of a few hundredths microns. These alpha particles may reach sensitive bone cells and eventually cause osteosarcomas. In the case of beta emitters such as strontium 90, beta rays are less energetic but travel a longer path. They can reach as far as the bone marrow and eventually cause leukaemia.
© IN2P3
Like radioactive activity, potential radiotoxicity is a useful risk indicator. However radioactive elements must enter in our body to exercise their damages, like Locust’s poisons, the famous roman poisoner. Fortunately the chances of this happening are, barring accidents, very small. The qualifier « potential » beeing generally forgotten, potential radiotoxicity is often mistaken for a true toxicity, generating misunderstandings and fears.
Ingestion or inhalation of a radioactive element results in a so-called committed dose, because its effects are oncoming and would be spread over long periods of time, may be up to our whole lifetime. One must take into account how radioactive materials are eliminated, how they are fixed by our body, the type of radiation and the radioactive decay half-life.
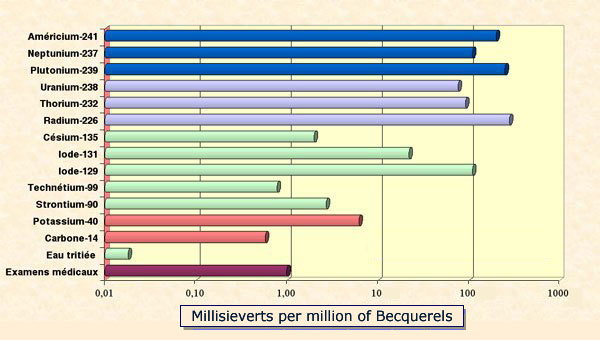
Hierarchy of doses factors
Dose factors are used to convert activity doses into biological doses, converting becquerels (Bq) into millisieverts (mSv). The graph shows the doses resulting from the absorption of 1 million becquerels of several radioelements. A logarithmic scale is necessary to represent the great variation of resulting doses.The alpha emitters (in blue) are by far the most toxic elements to absorb. A few fission products are shown in green. Two natural radioelements, potassium 40 and radiocarbon, present in the human body are in pink. The bottom line gives for comparison the average annual dose resulting from medical exams – around 1 mSv.
© IN2P3
There is a wide hierarchy in radiotoxicities. Tritium is the least radiotoxic radioisotope. On the contrary, most of the heavier nuclei, uranium, plutonium and minor actinides are very radiotoxic because they emit short range alpha particles. Plutonium-239 is thus 14,000 times more radiotoxic than tritium.
The very low nuisance tritium nuisance is due to the particularly small energy of its beta electrons (0.018 MeV maximum) and to the fact that this isotope of hydrogen is usually reliminated from the body before having disintegrated (its 10 days biological half-life is much shorter than its 12 years radioactive half-life). Instead, the plutonium is radiotoxic because this alpha emitter can be fixed in the bones and liver for a long time. Fortunately, plutonium and minor actinides atoms have a very low mobility in nature, which reduces their likelihood of being ingested or inhaled by humans.
NEXT : Dose factors
Other articles on the subject « Radiation Effects »
Deterministic effects
The domain of strong doses and severe, reproducible effects For high doses above a certain thresh[...]
Probabilistic effects
The domain of low and average doses For weak or medium-strength doses, the effects are not as cle[...]
Dose-effects Relationship
Can effects caused by low doses of radiations be predicted ? Even though we are constantly expose[...]
Cumulative Dose
What is the cumulative exposure? We receive an average of 2.5 millisieverts (mSv) per year from n[...]
Dose Rate
Acute and chronic exposures The « effective dose » is not sufficient by itself to characterize on[...]
Hiroshima Nagasaki Survivors
An exces of cancers and leukaemia among survivors Our knowledge of the risks of cancers due to ra[...]
Linear No-threshold Model
A Precautionary Principle applied to radioactivity … Is it possible to predict the effects [...]
Low doses effects
Do low doses of radiation have any effect? Radioprotection experts commonly refer to doses below [...]
Radiosensitivity
Sensitivity of DNA in living cells to radiation Although we still know little about the effects o[...]
Dose Factors
What doses when swallowing or inhaling radioactive atoms ? How to evaluate the doses resulting fr[...]