Charged Particle Effects
A gradual loss and transfer of energy
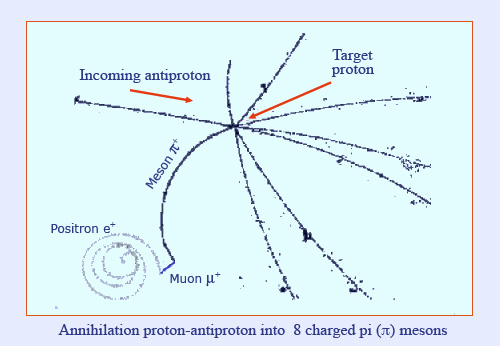
A continuous transfer of energy
This ‘bubble chamber’ image shows charged particles gradually depositing their energy along their trajectory. Bubble chambers were large detectors built in the 1960’s and ‘70’s and installed on the trajectories of high energy particles produced by large accelerators. This particular image shows the annihilation of an antiproton coming from the left with a target proton in the liquid hydrogen filling the chamber. The antiproton and the eight particles produced by the annihilation ionize the liquid nitrogen, causing it to heat up and produce microscopic bubbles which allow for identification.
© IN2P3
Alpha rays, fission products ; heavy, slow and ionizing particles
Contrarily to neutral particles, electrically charged particles lose energy progressively as they travel: by liberating electrons from the atoms that they come across. They gradually slow down and then come to a complete stop. Alpha particles are small nuclei whose energy is not high enough (with a few exceptions) to induce a nuclear reaction. They interact essentially with the medium they pass through because of their electric charge. They are strongly ionizing and easy to stop, but are much more dangerous than beta rays in case of ingestion or inhalation.
Nuclear fission that takes place in nuclear reactors produces also charged nuclei (known as fission products) that inherit most of the liberated energy. This energy will slowly be dissipated through interactions with atoms along a very short trajectory. Heavier than alpha particles, fission products have a proportionally greater electric charge. They are extremely ionizing. Each of them take away an energy some 25 times higher than the 4 MeV given to an alpha particle. This energy is converted into heat, which is at the origin of the electricity produced by nuclear reactors.
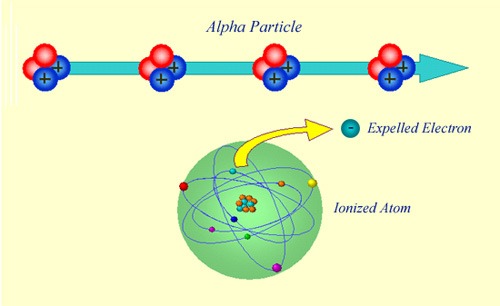
A relatively long range action
As a result of its positive electric charge, an alpha particle is capable of attracting electrons from a relatively large distance. Pulling electrons away from many atoms, the alpha particle loses energy, slows down and finally stops. The atoms that have lost electrons are known as ‘ions’, carrying a positive electric charge. As a result, the phenomenon is known as ‘ionisation’.
IN2P3
Beta rays : light electrons, low ionizing relativistic partucles.
Electrons or positrons also interact with the medium through their electric charge, which is far smaller than that of a nucleus. While alpha particles travel in short, straight lines, beta particles (some 8,000 times lighter) have long, unpredictable, chaotic trajectories with numerous abrupt changes of direction.
As a result of their low mass, beta particles are faster than their alpha cousins. An electron with an energy of 20 keV, considered as low, can travel at 82,000 kilometres per second, while an electron with 1 MeV (1000 keV) of energy can go as high as 282,000 kilometres per second – almost the 300,000 kilometres per second that light can travel in vacuum. When electrons travel through a transparent medium, such as glass or water, they can go faster than the speed of light for that specific medium. As a result, they emit a characteristic type of light known as ‘Cherenkov radiation’.
These electrons are known as ‘relativistic’. A complete description of their behaviour requires knowledge of Einstein’s theory of relativity. Highly relativistic electrons and positrons which pass near a nucleus experience another phenomenon known as ‘Bremsstrahlung’ or ‘braking radiation’. Under the influence of the nucleus’s intense electric field, these particles emit a gamma or X- photon which takes away some of their energy. As a result, the electron slows down. ‘Bremsstrahlung’ photons that accompany beta radiation need to be considered in radioprotection.
Other articles on the subject « Radiations effects in matter »
Alpha Rays in Matter
An atomic bulldozer, strongly ionizing along a very short path Alpha particles are simultaneously[...]
Beta Rays in Matter
Light electrons : a chaotic journey through matter Beta electrons and positrons have equal and op[...]
Bremsstrahlung
A relativistic phenomenon that applies to electrons and positrons… The phenomenon of bremss[...]
Neutral Particle Effects
Energy transfer by proxy… Neutral particles that are of interest in the field of radioactiv[...]
Cherenkov Effect
When an electron goes faster than light in air and water … The Cherenkov effect occurs when[...]
Cross Section
Cross section or the interaction probability of a particle Cross section is the name given by phy[...]
Gamma Rays in Matter
Gamma can be attenuated but never fully stopped The neutral gamma rays leave very different effec[...]
Photoelectric Effect
The most effective mechanism of photon absorption The photoelectric effect is the phenomenon that[...]
Compton Effect
Photons as projectiles and electrons as targets The Compton effect is the name given by physicist[...]
Macroscopic Effects
Effects on inert or organic matter The ionisation of atoms surrounding the trajectory of an alpha[...]