When radiation passes through matter …
The effects of a particle travelling through matter depend on its nature and the environment encountered. The electric charge of the particle or its absence determines the type of interaction or behavior.
A charged particle passing through the electronic clouds of atoms would contineously tear away some of their electrons, a phenomenon called « ionization » . Neutral particles – gamma or neutron – do not interact in a progressive manner. They set in motion charged particles : a gamma would knock on an atomic electron, a neutron a proton or a light nucleus. These secondaries particles will then ionize the atoms. Ionised atoms generally reorganize themselves by emitting photons, among them characteristics X-rays.
The mass of the incoming particle also plays an important role; alpha particles, which are comparatively heavy, are capable of stronger “ionisation”, but are slowed down much more quickly. Beta particles which are themselves electrons and several thousand times lighter, are able of travelling longer distances, but without achieving the same ionising effect. The movement of charge can also be achieved by neutral particles, such as neutrons or energetic photons, colliding with charged particles, thus achieving an indirect ionisation.
The alpha, beta and gamma rays of radioactivity have not enough energy to make matter radioactive. On the contrary neutrons that intrude easily inside the nucleus can provoke nuclear rexctions and induce radioactivity.
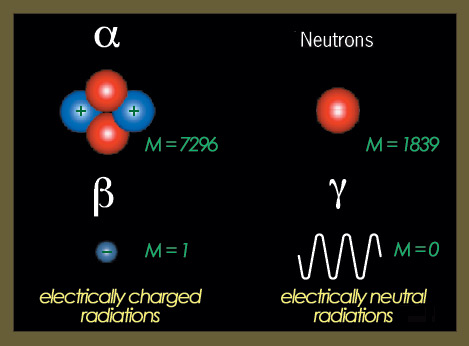
The four types of radiations
The effects of particles (or radiations) depend primarily on their charge and mass. 1) Electrically charged particles (alpha and beta) lose energy by ionising atoms they come in contact with, whereas neutral particles (neutrons or gamma rays) lose their energy by colliding with electrons or nuclei and cause indirect ionisation. 2) The masses of the different types of radiation are substantially different, with the alpha particle weighing nearly 7,300 times more than the beta particle (either an electron or a positron). 3) Neutrons and, to a lesser degree, alpha particles, are the two types of radiation made of nucleons able to produce changes within the nucleus.
© IN2P3
The effects of radiations are not limited to ionisation, however. By changing the electron balance, incoming rays can disturb the atomic structure and cause molecules to heat up or even break apart. The eventual results can differ, depending on a host of different properties; such as whether the exposed material is solid or gaseous, how complex its constituent molecules are, or how regular its structure is. Such effects are particularly relevant in discussing the exposure of living matter.
As radiation has the capability to destroy living cells, such an exposure can be beneficial if either diseased or harmful cells are targeted. The effects can also be deadly for the living being if its healthy cells suffer prolonged exposure, though most cells have the ability to repair the damage caused by light particles. Judging the effect of radioactivity on any given individual is incredibly complex, given the high number of considerations involved, but high doses are, in almost all cases, deadly.
Understanding the way in which radiation interacts with matter allows us to protect ourselves from the harmful effects. The simplest way to do this is by placing a protective shield around the radioactive source; alpha particles can be stopped by a layer as thick as a sheet of paper, beta radiation cannot penetrate more than a few centimetres of aluminium, whereas gamma rays are almost impossible to stop. In all three cases, however, the layer can be designed so as to minimise the risk to all living matter present. Even the extremely radioactive substances used in nuclear reactors can be rendered virtually harmless by being surrounded by several metres of water.
Articles on the subject « Radiations effects in matter »
Alpha Rays in Matter
An atomic bulldozer, strongly ionizing along a very short path Alpha particles are simultaneously[...]
Beta Rays in Matter
Light electrons : a chaotic journey through matter Beta electrons and positrons have equal and op[...]
Bremsstrahlung
A relativistic phenomenon that applies to electrons and positrons… The phenomenon of bremss[...]
Neutral Particle Effects
Energy transfer by proxy… Neutral particles that are of interest in the field of radioactiv[...]
Cherenkov Effect
When an electron goes faster than light in air and water … The Cherenkov effect occurs when[...]
Cross Section
Cross section or the interaction probability of a particle Cross section is the name given by phy[...]
Gamma Rays in Matter
Gamma can be attenuated but never fully stopped The neutral gamma rays leave very different effec[...]
Photoelectric Effect
The most effective mechanism of photon absorption The photoelectric effect is the phenomenon that[...]
Compton Effect
Photons as projectiles and electrons as targets The Compton effect is the name given by physicist[...]
Macroscopic Effects
Effects on inert or organic matter The ionisation of atoms surrounding the trajectory of an alpha[...]