The best known of elementary particles
The electron is an elementary particle that plays a fundamental role in all branches of science and in everyday life. Its discovery in 1897 by the English physicist Joseph John Thomson marked a milestone in our understanding of Nature. It was followed a few years later by the discovery of radioactivity.
Thomson was investigating the nature of « cathodic rays » which were emitted by the cathode of cathodic ray tubes, the ancestors of our television sets before the arrival of flat TV screens.
From his observations, Thomson deduced the corpuscular nature of cathode rays. He measured the ratio of the mass and charge of these particles and deduced that they were at least 1000 times lighter than the lightest object known at the end of nineteenth century, the hydrogen ion (in fact a proton).
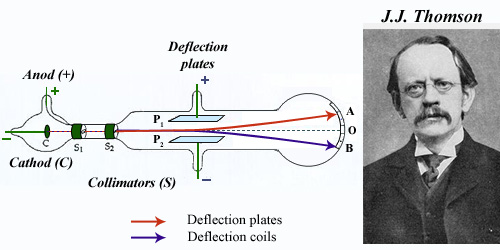
1996 : the electron is discovered by J.J. Thomson
Vacuum was made in Thomson’s cathode ray tube. The cathode C was set to a negative voltage of a few hundred volts, the anode beeing grounded. Cathodic rays moved towards the anode passing through two collimators S and hit the tube bottom at the point O. By applying an electric field between the two plates P1 and P2 cathodic rays were deflected along the path indicated in red. They were also deflected by the magnetic field of a coil (blue line) . From these observations and by measuring these deflections, Thomson was able to deduce the corpuscular nature of cathodic rays and identify them with tiny particles he named electrons.
© JJ. Thomson
The electron is still lighter than measured by Thomson : its mass is only one thousand eight hundred thirty-seventh of that of a proton. The electronic cloud in the atom weighs even less compared to its nucleus which is burdened by the presence of neutrons. Because masses of particles measured in grams are so tiny, nuclear physicists prefer to use instead mass energies E = mc² (its mass multiplied by the square of the speed of light c). The electron mass energy is 511 keV.
The electric charge of the electron is the smallest electric charge known. By convention, it was chosen negative. Its value noted e is equal to 1,6 to the power -19 Coulomb (1,6 preceded by 19 zeros). All the electric charges, excluding the ones from the quarks, are positive or negative multiples of this charge e considered elementary.

Electron mass and electric charge
The electron mass and electric charge – expressed in gram and coulomb as units – are given by exceedingly small numbers; 0.911 and 1.6 preceded by 27 and 19 zeros.
© IN2P3
Today, the electron appears as one of the elementary constituents of matter, like the quarks called up and down, which are the constituents of protons and neutrons. The electron, with these two quarks and the electron-neutrino belongs to the first generation of the fundamental constituents of matter.
The electron, like the quarks and the neutrino, may be viewed as the smallest spinning-tops that can be imagined. They can have only two states of spin or rotation, the intensity of this rotation being unique and the smallest known. The great italian physicist Enrico Fermi formulated the theory of these corpuscles with two spin states like the electron. but also the quarks, protons and neutrons. They are called fermions in his honor.
Fermions are subject to a rule called the Pauli exclusion principle. The Pauli exclusion principle says that two electrons can not be in the same state (called quantic state). This rule plays a major role in the atom. Without it, the atomic cloud of electrons, attracted by its electric positive charge, would crash onto the atom nucleus.
According to the Pauli principle, only two electrons can share the territory closest to the nucleus : one spinning in one direction, the second in the other, because they are distinguished by the direction of rotation. A third electron, can not share this space, as both spin states are taken. He must choose a more remote area. This territory farther – the atomists call it layer L – accommodates up to 8 electrons. So, layer after layer, the atoms we know are built with a voluminous cloud of electrons surrounding a small nucleus …
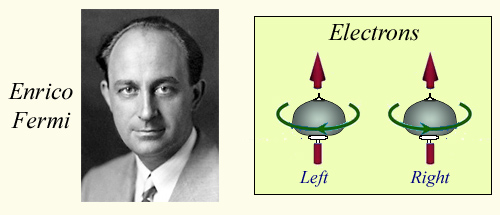
Elecron spin
Enrico Fermi ( 1901-1954) and Paul Dirac (1902-1984) formulate the theories of the behaviour of fermions, these corpuscles which could have only two states of rotation or spin with respect to an axis ; one called left handed, the other right handed. Electrons can not be in the same ‘quantic state’ according to the Fermi-Dirac theory. They organize themselves to form voluminous clouds around atomic nuclei.
© IN2P3
Electrons do not content themselves with transporting electricity. They are everywhere, playing an active role, in physics, chemistry, biology. It is by sharing their peripheral electrons that atoms assemble into molecules. Almost everything in chemistry is a matter of electrons.
In the field of radioactivity, electrons play an active role in three phenomena, the most important beeing the first :
– Beta radioactivity, the emission of an electron by the nucleus ;
– Electron capture, the capture of an atomic electron by the nucleus ;
– Internal conversion, the ejection from an atom of one of its electrons by a gamma emitted by the nucleus.
Other articles on the subject « The atomic world »
The Atom
An almost empty space with mass concentrated in a tiny nucleus The atom is often viewed as a mini[...]
Atomic Energy Levels
A shell structure …. The conquest of space has familiarized us to the concept of a satellit[...]
Photons
The elementary components of light and electromagnetic waves Light is composed of infinitesimal i[...]
Orders of Magnitude
The very small and the very large … The atom, and the nucleus in particular, belong to the [...]
Avogadro’s Number
Trillions of billions of very small atoms …. The microscopic size of atoms comes with their[...]
E=MC²
The Einstein formula, a relation between mass and energy The energy released by a chemical reacti[...]