The positive electron, the first ambassador of antimatter
The positron was discovered in 1933 by the American physicist Carl Anderson. Anderson was studying the cosmic rays particles with a Wilson chamber or cloud chamber. Steam condensed into fine droplets along the trajectory of electrically charged particles crossing the chamber, visualizing this trajectory. A magnetic field produced by a coil bent trajectories which allow to determine the sign, positive or negative, of the particle electric charge.
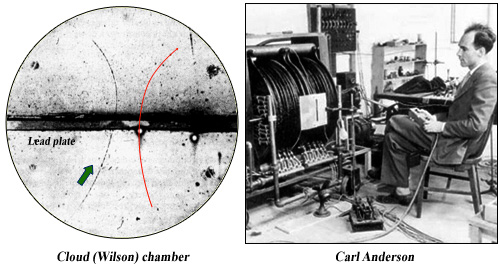
Anderson positron
Historical photograph of one of the first positron trajectories observed by Anderson in 1933 in a cloud chamber. A magnetic field deflect the particle trajectory and the insertion of a lead plate allow to determine the direction of its movement (arrow). The deflection indicates a positive particle (an electron would have followed the trajectory in red) which could not be a proton whose path would be much shorter.
© C.D. Anderson, Physical Review 43, 491 (1933).
Within the atom, the role assigned by Nature to positive protons and negative electrons could not be more different despite the fact they carry the same elementary electric charge +e or -e. The « positive » proton is confined inside the nucleus : The « negative » electron belongs to the electron flowing around the nucleus. Protons are 1837 times heavier. Why such an asymmetry ? Why would not exist in Nature negative protons and positive electrons ?
The discovery of a positive electron by Anderson was the first evidence of these particles symmetrical to the particles of our everyday world. They belong to what is now called antimatter.
To demonstrate the existence of the antiproton, physicist had to wait the development of large particle accelerators. In 1955, the team of Emilio Segre and Owen Chamberlain using the Bevatron, the new accelerator at Berkeley (California), proved the existence of the antiproton, and then shortly afterwards that of the neutron antiparticle, the antineutron.
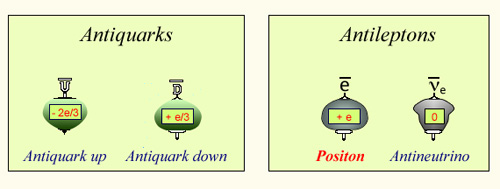
The 4 fundamental constituents of antimatter
To the four fondamental constituents of ordinary matter, corresponds in antimatter four constituants which are their antiparticles. The first one, the positron (or positon) is the more visible. The antineutrino, produced abundantly in beta-minus decays, but virtually undetectable. The antiquarks are the constituents of antiprotons and antineutrons, antiparticles of nuclei protons and neutrons.
© IN2P3
The positron does not exist in our environment. According to the Einstein formula E = M c² relating mass and energy, it is possible to produce positrons with an energy greater than 511 kEv, the mass energy of the positron or electron. One should create simultaneously one antiparticle, either an electron or a neutrino. In the process, the total electric charge should be conserved.
Some positrons are generated by a rare type of radioactive decays, beta-plus decays. The positron is produced together with a invisible neutrino-electron that escape detection. Energy is taken from the energy released in the decay.
A second process is the simultaneous production of an electron and a positron during the interaction of an energetic gamma with a nucleus. As an electron is also produced, one should add 511 kev to the 511 kev needed to create the positron : the gamma energy should be larger than 1022 keV. Few natural gamma in radioactive decays have such energies. Pair production plays a marginal role in our environment.
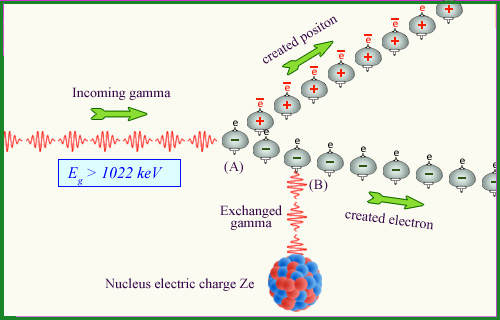
Positron-electron pair production
Quantum Mechanics allows ultra shorts transformations of a gamma into a pair of particle-antiparticle, such as an electron and a positron. If during this short instant, the pair finds itself in the vicinity of the strong electric field of a nucleus it interacts with it (B). The interaction with the nucleus enables the creation of the electron and positron to remain permanent. The « mass energy » (twice 511 keV) necessary for the formation of the two particles is taken from the energy of the gamma that must be must greater than a 1.022 MeV threshold.
© IN2P3
Every kilogram of the matter in our world contains billions of billions of billions of electrons. If it does not travel in vacuum, a positron quickly encounters one of these electrons. When they meet, the positron and the electron, which are Antiparticles of each other, destroy themselves mutually, they annihilate. Two annihilation gamma with equal energy are also emitted back to back. They carry each 511 keV, that is the mass energy of the two particles which is thus restored. This characteristic annhihilation reaction is used in nuclear medicine for the screening of cancers.
Moving in the midst of its countless electron enemies, positrons are virtually absent from our environment. The same happens to antiprotons. How to explain this lack of antimatter around us, while at the elementary level whenever a particle is created or destroyed, an antiparticle is created or destroyed too. Where is the antimatter? This is one of the major questions addressed particle physics.
Experiments have shown that a perfect symmetry between particles and antiparticles is not perfectly respected in the field of the weak forces responsibles in particular of beta radioactivity. Can this asymmetry, which is very small, be the explanation of antimatter absence around us ?
Other articles on the subject « Alpha Beta Gamma rays »
Alpha (α) radioactivity
How heavy nuclei lose weight … by emitting alpha particles Alpha (α) radiation was first ob[...]
Beta (β) radioactivity
How Nature corrects an excess of protons or neutrons Beta (β) radioactivity was first observed in[...]
Beta spectrum
Beta electrons do not have a unique characteristic energy Three particles, the recoil nucleus, th[...]
Electron Capture
A minor mode … competing with positron emission Electron capture is a comparatively minor d[...]
The electron-neutrino
An electron that would have lost its electric charge The easiest way to conceive a neutrino is to[...]
Muons
An heavy electron abundant in cosmic rays Muons were first observed in 1936 by the Americans phys[...]
Radioactivity Gamma (γ)
How nuclei get rid of excess energy It was in 1900 that the French physicist Paul Villard first f[...]
Nuclear Desexcitations
Gamma are the light emitted by atomic nuclei A french proverb used to say « all roads go to Rome.[...]
Internal Conversion
When a gamma expells an atomic electron and is absorbed Internal conversion is a nucleus desexcit[...]
Nuclear Transmutations
The ancient dream of the alchemists… The alchemists of the Middle Ages and the Renaissance [...]