How Nature corrects an excess of protons or neutrons
Beta (β) radioactivity was first observed in the form of a mysterious ray that was deflected by electromagnetic fields in the opposite direction from alpha radiation. The radiation was therefore known to consist of negatively charged particles. After J.J. Thomson discovered (a little before in 1897), the fundamental carrier of negative electric charge, the electron, beta radiation was quickly found to be made up of the same particles.
Physicists had to wait the discovery in 1932 of the positive electron called a positron, followed by that of artificial radioactivity in 1934, to observed similar decays carried by positive electrons. These two variants of beta radioactivity variants are called beta-minus radioactivity and beta-plus radioactivity.
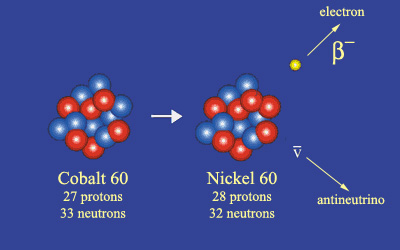
Example of a beta-minus decay
A cobalt 60 nucleus, containing 33 neutrons and 27 protons, has an excess of 6 neutrons – shown in blue. To even the balance, one of these neutrons transforms into a proton (shown in red) to form a stable nucleus of nickel 60 with 28 protons (one more than before) and 32 neutrons (one fewer than before). At the time of the decay, two other particles are created: an electron and an antineutrino which is able to pass undetected.
© IN2P3
At the origin of this type of radiation is a force within the nucleus capable of transforming one type of nucleon into another (a proton into a neutron, or vice versa): the so-called ‘weak‘ forces. This transformation does not change the total number of nucleons, but is accompanied by the emission of an electron (or a positron) to compensate the change of electric charge. The electron is expelled together with a kind of neutral positron – an antineutrino – while a positron is expelled with a neutrino, the neutral counterpart of the electron.
Beta decays are observed in Nature, when the process releases energy, which is the case for beta emitters. The alternative to correct an excess of one type of nucleons – the direct expulsion of a proton or neutron from the nucleus – costs generally energy and occurs only for very unstable nuclei produced in reactors with a large excess of neutrons.
Beta-minus radiation, the emission of an electron and an anti-neutrino, occurs when a neutron transforms into a proton. The reverse process, whereby a proton becomes a neutron through the emission of a positron and a neutrino, is the source of beta-positive radiation. The neutrinos and antineutrinos are tiny, almost massless particles that are virtually impossible to detect.
The kinetic energy released in beta decay is shared among the three bodies involved: the recoiling nucleus, the electron (or positron) and the antineutrino (or neutrino). The nucleus, whose mass is by far the greatest, takes away comparatively little energy. The two light particles share practically the energy released in the decay. The electron (or positron) usually takes away a little less than half of this energy.
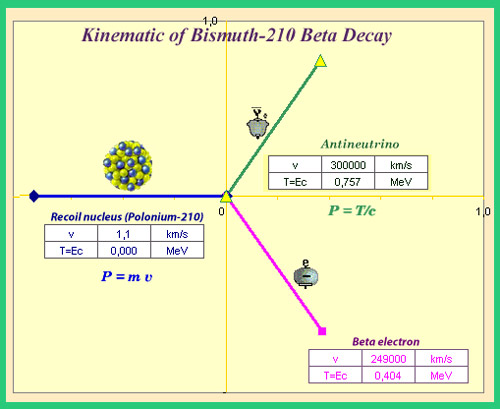
Three-body beta kinematics
When beta decay takes place, the energy released is shared between the recoiling nucleus, the electron and its antineutrino. The nucleus being initially at rest, the sum of the three momentum vectors must be equal to zero (for the nucleus, the momentum is equal to the mass times the velocity v). Shown above case where all three momenta have equal valueswith a 120° angle . The kinetic energy T and speed of the recoiling nucleus are negligible compared to the two other particles, which shared the decay energy (1.16 MeV in the decay of bismuth 210).
© IN2P3
A proton excess is rare in nature, and we are indebted to Irene and Frederic Joliot-Curie for synthesizing the first beta-plus emitters after their discovery of artificial radioactivity in 1934. Today beta-plus emitters are produced by small accelerators such as cyclotrons for medical applications. Positrons are particularly effective in carrying out positron emission tomography. The main application is that of fluorine-18 which is used in positron emission tomography for cancer screening.
The radioactive half-lives of beta emitters are much shorter, with a few exceptions, than half-lives of alpha emitters. Sometimes they can be very short. Energies released in beta decays vary from a few keV in the case of tritium to around 1 MeV. They are furthermore shared between electrons and antineutrinos. They are far below the energies of alpha particles that are above 4 MeV.
A few beta-emitters exist in nature, tritium and carbon-14 produced in the atmosphere by cosmic rays or potassium-40 a long-lived isotope of potassium responsible of 4000 decays per second in the human body. Others, such as bismuth-210 are descendants of uranium and thorium nuclei. In reactors, the products of nuclear fission that inherit of the neutron excess in uranium or plutonium nuclei are also beta emitters. The best known are iodine-131 and caesium-137.
Other articles on the subject « Alpha Beta Gamma rays »
Alpha (α) radioactivity
How heavy nuclei lose weight … by emitting alpha particles Alpha (α) radiation was first ob[...]
Beta spectrum
Beta electrons do not have a unique characteristic energy Three particles, the recoil nucleus, th[...]
Electron Capture
A minor mode … competing with positron emission Electron capture is a comparatively minor d[...]
Positron
The positive electron, the first ambassador of antimatter The positron was discovered in 1933 by [...]
The electron-neutrino
An electron that would have lost its electric charge The easiest way to conceive a neutrino is to[...]
Muons
An heavy electron abundant in cosmic rays Muons were first observed in 1936 by the Americans phys[...]
Radioactivity Gamma (γ)
How nuclei get rid of excess energy It was in 1900 that the French physicist Paul Villard first f[...]
Nuclear Desexcitations
Gamma are the light emitted by atomic nuclei A french proverb used to say « all roads go to Rome.[...]
Internal Conversion
When a gamma expells an atomic electron and is absorbed Internal conversion is a nucleus desexcit[...]
Nuclear Transmutations
The ancient dream of the alchemists… The alchemists of the Middle Ages and the Renaissance [...]