A variety of radioactivity with a multitude of uses and properties
The description of all currently-existing nuclei as ‘naturally-occurring’ is a fallacious one. In reality, all nuclei that existed, exist, or could exist can be said to be naturally-occurring. The erroneous distinction dates back to 1934, when Frédéric and Irène Joliot-Curie discovered a technique to recreate nuclei that were once naturally present, but had disappeared due to their radioactive decays. As a result, all these nuclei when recreated by man, have been referred to as “artificial”, though the term is somewhat misleading.
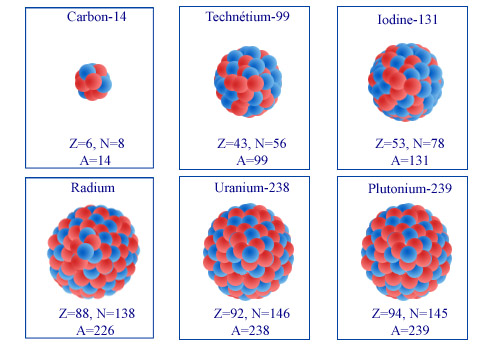
The diversity of radioactive nuclei…:
Three naturally-occurring nuclei (carbon-14, radium-226 and uranium-238) and three nuclei that have been artificially produced (technetium-99, iodine-131 and plutonium-239). The three nuclei of the top row are beta emitters, while the three of the bottom row emit alpha particles.
© IN2P3
There are 287 different nuclei that can be found in nature. The total number of “artificial” nuclei, however, is currently over 2500; a number that keeps on growing as experiments continue. Some of these created nuclei are so unstable that they decay before having time to attract any electron, and thus cannot even be considered as real ‘atoms’.
Very few naturally-occurring nuclei and all lab-created nuclei are radioactive. Among them are a few celebrities: uranium used in nuclear reactors, Marie Curie’s radium, carbon-14 employed by archaeologists, radon the main source to exposition to radioactivity, potassium-40 present in the human body and tritium an heavy isotope of hydrogen. These are all radioactive nuclei that are found in nature. In contrast to these we have the famous artificial nuclei; the much-prized and much-feared plutonium, cobalt-60 with its many uses, technetium and thallium.
Nuclear fission is a process often used to to produce radioactive nuclei. A well-known fission product, Iodine-131, was also released by the Chernobyl disaster and believed to be responsible of thyroid cancers. Another product of nuclear fission, caesium-137, is today the main legacy of the radioactivity released into the atmosphere during the Tchernobyl and Fukushima accidents and by nuclear tests in the 1960s. It is used also as a radioactive tracer.
Articles on the subject « Main Radioactive Nuclei »
Plutonium 239
Plutonium 239: an artificial fissile nucleus, highly sought-after and feared Plutonium, the ninet[...]
Plutonium Properties
A transuranic element with long-lived radiotoxic isotopes Plutonium is a very dense metal, radioa[...]
Radium
The radioactive nucleus that made History Radium is an extremely rare element that was first disc[...]
Carbon-14
A by-product of cosmic rays Carbon-14 (C-14) formation in the atmosphere The nucleus of carbon-14[...]
Potassium-40
A curiosity of Nature and a very long lived beta emitter Potassium 40 is a radioisotope found in [...]
Iodine 131
Radioactive iodine : A dangerous and short lived fission product Iodine 131 is a radioisotope wit[...]
Tritium
A radioactive isotope of hydrogen Tritium is a beta-emitting radioactive isotope of hydrogen. Its[...]
Caesium 137
A legacy of atmospheric nuclear bomb tests and accidents Caesium 137 is a radioactive element wit[...]
Strontium-90
A fission product with properties close to calcium Strontium-90 is with cesium-137 a major radioa[...]
Technetium 99
A pure gamma emitter widely used in nuclear medicine Of all the atoms below uranium in Mendeleyev[...]