A shell structure ….
The conquest of space has familiarized us to the concept of a satellite orbiting around our planet. Booster rockets can change the energy or trajectories of a satellite at will, and have the ability to transfer a satellite to any other orbit.
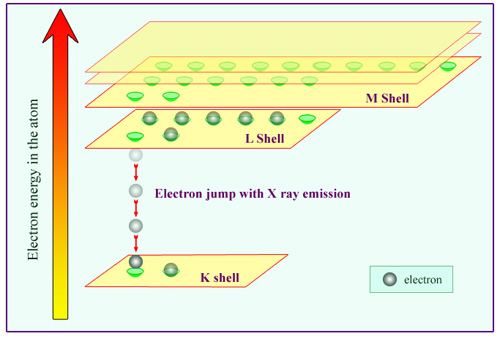
Energy levels of atomic electrons
This diagram of the energy of electrons in an atom shows its layer structure. Quantum mechanics allows only a limited number of energy values or levels. This is very different from the classical physics, where any energy value would have been allowed. The lowest energy level (the K shell) can only be occupied by two electrons, the second level (L shell ) by 8 and the third (M shell) by 18. The K shell electrons are closest to the nucleus. On the figure, an electron from the K shell has been ejected by the passage of radiation. The available space is immediately occupied by an electron from the L shell, which « jumps » to the lower level by emitting an X-ray.
© IN2P3
The electrons travelling around nuclei do not have the same sort of freedom. Nature forces electrons to have one of a limited range of energy values, in the same way that mechanics are forced to use screws that have a limited number of head sizes. Electrons can’t have whatever energy values they want, and each value is associated with a specific orbit trajectory. Photon emission and absorption play the same roles as booster rockets, allowing the electron to jump from one predefined ‘orbit’ to another.
Physics on the microscopic scale is governed by the laws of quantum mechanics, where it is preferable to talk about electron ‘states’ rather than ‘orbits’. Each state defines the energy an electron has, and the amount of space it takes up. The number of energy states an electron can occupy around a nucleus is also limited, and different states of the same energy are known as ‘shells’. The electrons of an atom belong to different shells characterised by their energies. These shells are known as K, L, M, N… by atom physicists.
The K shell, which feels the strongest attraction from the nucleus, is the first to fill up completely. Two electrons at most can fill this layer, and an atom third electron would have to go into the 2nd L shell. Electrons in the outer shells are more weakly attracted by the nucleus.
The binding energies of the electrons go down as the shell number increases. When a place on an internal energy shell is made available, an electron situated on a higher shell fills the gap by jumping to fill the place left over, increasing the strength of its bond to the nucleus. These transitions are accompanied by the emission of ‘electromagnetic’ energies defined by the difference in energies of the two shells. These tiny electromagnetic waves are photons which are sometimes capable of leaving an impression on our retinas.
The energies of these photons are unique, as the energies of the shells are accurately defined by the laws of quantum physics. The wavelength of the photons (which is to say their colour), linked as they are to their energies, are also unique. The photons emitted by an atom thus have mathematically-defined energies, wavelengths and colours.
The light emitted is characteristic both of the atom and of the shell energy jump. When this light is analyzed according to its wavelength by a prism, we observe a series of rays characteristic of the different energy jumps possible. These rays can be used as an identification mechanism, allowing physicists to check for the existence of specific atoms in distant stars by searching for these ‘fingerprints’.
Other articles on the subject « The atomic world »
The Atom
An almost empty space with mass concentrated in a tiny nucleus The atom is often viewed as a mini[...]
The electron
The best known of elementary particles The electron is an elementary particle that plays a fundam[...]
Photons
The elementary components of light and electromagnetic waves Light is composed of infinitesimal i[...]
Orders of Magnitude
The very small and the very large … The atom, and the nucleus in particular, belong to the [...]
Avogadro’s Number
Trillions of billions of very small atoms …. The microscopic size of atoms comes with their[...]
E=MC²
The Einstein formula, a relation between mass and energy The energy released by a chemical reacti[...]