A minor mode … competing with positron emission
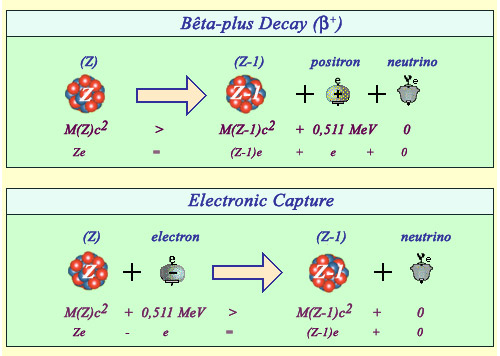
Positron emission versus electron capture
The emission of a positron and the capture of an electron are twin reactions both resulting in the diminution of the number of protons by 1 (from Z to Z-1) and the production of a neutrino.The positron observed in the final stage of the beta decay (top) is a new particle requiring the 0.511 MeV of its rest mass energy to be created. There is no such energy threshold in the case of electron capture (bottom). In both cases, practically all the enegy released is carried by light particles.
© IN2P3
Electron capture is a comparatively minor decay mode caused by the weak force. The best-known example is of potassium 40 : 11% of the nuclei of that isotope of potassium present in our body decay by electronic capture.
The electron capture trigger the emission of an invisible neutrino by the nucleus.
The capture of an electron has the same effect on a nucleus as the emission of a positron: one of its protons transforms into a neutron, diminishing the global electric charge of the nucleus by 1 unit. Electron capture, along with beta-positive decay, is Nature’s way of guaranteeing that no nucleus becomes too proton-heavy.
Ordinary beta-minus decay has no competitor on Earth however to reduce an excess of neutrons, since the capture of positrons would occur in an world made of antimatter.
The captured electron belongs to the group of electrons orbiting around the nucleus. Such captures turn out to be difficult. Most of the electrons orbit the nucleus at distances large compared to the nucleus. Even the innermost K-layer electrons are far from the very small volume of the nucleus where the weak forces responsible for the capture operate and transform the electron into a neutrino. This explains why electron capture is difficult and therefore rare.
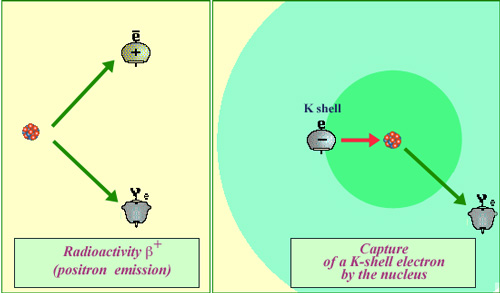
Electron capture : a difficult and rare process
Weak forces are behind positron emission and electron capture. Electron capture occurs much less frequently than the emission of a positron. Whereas beta decay can occur spontaneously when energetically allowed, for an electron capture the weak forces require that the electron comes into close contact with a proton of the nucleus. The probability that an electron, even one belonging to the inner ‘K’ shell, would find itself inside the nucleus is very low indeed (for potassium 40, the volume of the nucleus is less than a billionth of the K layer volume).
© IN2P3
However, electron capture is more economical in energy than positron emission, its competitor. The creation of a positron requires 511 keV, the mass energy of the positron. If the energy released in the decay is smaller than 511 keV, the emission of a positron (beta-plus decay) is not allowed. Below this energy threshold, electron capture becomes the only process available to reduce an excess of protons.
Electon captures often passes unseen, as the neutrino that carries away the released energy is impossible to detect. The recoiling nucleus also barely moves, with the few microns that it covers being too small to be observed.
These events would go unnoticed if it were not for the restructuring that the nucleus and electron shells both undergo. Electrons are usually captured from the inner K layer, leaving ‘holes’ behind them. An atom with a gap in its electron structure rearranges itself, emitting X rays in the process or Auger’s electrons. Such a capture may also leave the nucleus in an excited state, at a higher energy its ground state, causing it to release desexcitation gamma rays.
As a result, electron capture particular decay mode is very hard to detect. This particular decay mode was discovered only in 1937 by the American physicist Luis Alvarez (1911-1988), some forty years after the discovery of beta-negative radioactivity and only a few years after the observation of the positron and beta-positive decays.
Luis Alvarez, a physics Nobel laureate, had a long and brilliant carreer as a physicist. For instance, far away from electron capture, he proposed in 1980 a famous explanation of the dinosaurs extinction, suggesting it had been caused by an asteroid colliding with the Earth some 160 million years ago.
Other articles on the subject « Alpha Beta Gamma rays »
Alpha (α) radioactivity
How heavy nuclei lose weight … by emitting alpha particles Alpha (α) radiation was first ob[...]
Beta (β) radioactivity
How Nature corrects an excess of protons or neutrons Beta (β) radioactivity was first observed in[...]
Beta spectrum
Beta electrons do not have a unique characteristic energy Three particles, the recoil nucleus, th[...]
Positron
The positive electron, the first ambassador of antimatter The positron was discovered in 1933 by [...]
The electron-neutrino
An electron that would have lost its electric charge The easiest way to conceive a neutrino is to[...]
Muons
An heavy electron abundant in cosmic rays Muons were first observed in 1936 by the Americans phys[...]
Radioactivity Gamma (γ)
How nuclei get rid of excess energy It was in 1900 that the French physicist Paul Villard first f[...]
Nuclear Desexcitations
Gamma are the light emitted by atomic nuclei A french proverb used to say « all roads go to Rome.[...]
Internal Conversion
When a gamma expells an atomic electron and is absorbed Internal conversion is a nucleus desexcit[...]
Nuclear Transmutations
The ancient dream of the alchemists… The alchemists of the Middle Ages and the Renaissance [...]