Effects on inert or organic matter
The ionisation of atoms surrounding the trajectory of an alpha or beta particle in matter, or the interaction of a gamma ray with a nucleus or an electron, is a microscopic phenomenon. Any effects on the scales that we can see are the result of a very large number of these fundamental interactions.
By expelling electrons from the atoms they pass, the ionising particles cause a temporary instability in these atoms and molecules. The key role these electrons play in the molecular structure means that these expulsions cause disruptions. Free radicals (segments of molecules) are created in an extraordinarily brief lapse of time. Depending on the surrounding conditions, these highly active free radicals can either recombine or induce chemical transformations in the substance.
The macroscopics effects naturally depend on the dose given off and the area irradiated: living or inert, solid or liquid, organic material or not. The effects on humans have a special importance to us and deserve their own chapter. Our interest is only natural, as the alpha and beta particles have the ability to ionise our cells and potentially damage our precious DNA. Effects have been observed at doses in excess of one gray (Gy), and in radiotherapies cancerous tumours are exposed to doses of the order of 50 Gy for curative purposes. The possible effects of weak doses remain unclear despite the extensive investigations made.
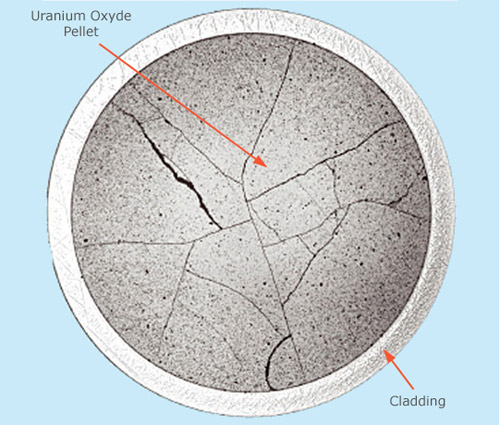
Damages of extreme doses to nuclear spent fuel
This microscopic scan of a pellet of nuclear fuel was taken after it had spent between 3 and 4 years inside a reactor. The exposure has already caused significant damage, as the cracks clearly show. These structural weaknesses are among the main factors which limit the amount of energy that can be extracted from these pellets. It must be remembered, however, that the damage made was caused by are extreme levels of exposure, tens of billions of joules per kilogram (also known as grays) that fission reactions produce in the uranium pellets. These
© CEA
Ignoring humans and living beings for the moment, the exposure of inert matter (such as food and drink) to radiation produces very little effect. There is, however, no reason not to expose such materials to very high doses.
The most extreme example is that of the nuclear fuel at the heart of a reactor, which receives the equivalent of several billion grays. Similar doses are also absorbed by those detectors placed at the heart of large experiments in nuclear or particle physics using high energy accelarators.
High levels of exposure can change the structure of matter, accelerate ageing processes or, in the case of crystalline structures, provoke structural deformation. It was the ignorance of these effects that led to the 1957 Windscale accident – the most severe nuclear accident in British history.
High doses, though nowhere near on the scales mentioned above, are often used in industry, agriculture and the food industry, as well as in hospitals and museums.
Beneficial changes to the molecular structure can be induced by carefully moderated exposure to radiation. These changes have been seen in a range of products that are in current use. Links in many polymers, for instance, have been altered by radiation to have specific characteristics – such as the ability to shrink under exposure to heat. These polymers are regularly used in the packing industry.

Getting rid of pests by sterilization
Doses of several hundreds of grays are used in the food business to destroy the microorganisms or, as can be seen in this picture, the insects which pose a threat to humans. The photograph shows male larvae of Mediterranean flies that have been exposed to a radioactive source of cobalt 60. The flies are thus sterilized by a dose of several hundred grays. Once released, the species’ ability to proliferate is dramatically reduced.
© AIEA
Exposure to radiation (principally gamma) is often used to disinfect and sterilise medical equipment, pharmaceutical or cosmetic products, and to help prolong the shelf-life of food. The irradiation of food leaves no lasting effect; it only carries enough energy to destroy bacteria and other microorganisms before disappearing.
NEXT : Conservation effects
NEXT : Disinfecting effects
Other articles on the subject « Radiations effects in matter »
Charged Particle Effects
A gradual loss and transfer of energy Alpha rays, fission products ; heavy, slow and ionizing par[...]
Alpha Rays in Matter
An atomic bulldozer, strongly ionizing along a very short path Alpha particles are simultaneously[...]
Beta Rays in Matter
Light electrons : a chaotic journey through matter Beta electrons and positrons have equal and op[...]
Bremsstrahlung
A relativistic phenomenon that applies to electrons and positrons… The phenomenon of bremss[...]
Neutral Particle Effects
Energy transfer by proxy… Neutral particles that are of interest in the field of radioactiv[...]
Cherenkov Effect
When an electron goes faster than light in air and water … The Cherenkov effect occurs when[...]
Cross Section
Cross section or the interaction probability of a particle Cross section is the name given by phy[...]
Gamma Rays in Matter
Gamma can be attenuated but never fully stopped The neutral gamma rays leave very different effec[...]
Photoelectric Effect
The most effective mechanism of photon absorption The photoelectric effect is the phenomenon that[...]
Compton Effect
Photons as projectiles and electrons as targets The Compton effect is the name given by physicist[...]