An equilibrium as old as the Earth
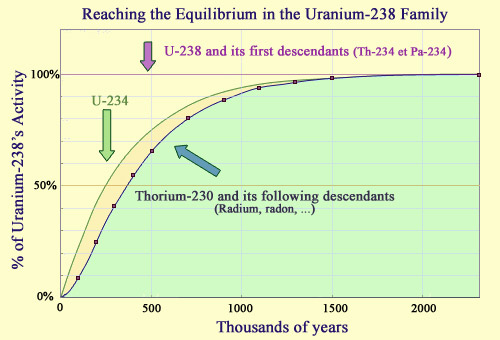
Establishing a radioactive equilibrium
In the uranium 238 lineage, the radioactive equilibrium takes about 2 million years to form, at which point the activities of these descendants become equal to that of uranium 238. The short half-lives of the first two descendants, thorium 234 and protactinium 234, mean that the first steps are reached quickly. It takes much longer for the next descendants, uranium 234 (half-life of 246,000 years) and thorium 230 (half-life of 75,000 years) to reach equilibrium. Radium and radon, the next descendants in the chain, have much shorter half-lives, and reach equilibrium at about the same time as the thorium-230. Two million years represent a brief instant for uranium 238, whose half-life is 4.5 billion years.
© IN2P3
Radioactive lineage is the term given to the chain of successive radioactive disintegrations that some nuclei undergo. In nature, radioactive lineage is particularly relevant for three heavy elements with half-lives of the order of billions of years: uranium 238, uranium 235 and thorium 232. The descendants of these three nuclei, present in trace quantities in rocks, make a substantial contribution to the natural levels of radioactivity.
Over the 4.5 billion years of the Earth’s history, all three of these decay chains have reached equilibria between the parent nucleus and the various descendants. The ratios evolve very slowly, ensuring that at any given moment the number of nuclei being formed is identical to the number of nuclei decaying. The activities are all practically invariant, and equal to the activity of the ‘ancestor’ nucleus.
The establishment of these so-called ‘secular’ equilibrium only takes place after a transition period. A classic example of this is uranium 238, an isotope that has a much longer half-life than any of the unstable nuclei it decays into. The decay of uranium 238 is so slow, in fact, that it is almost as though it was powered by a constant dripping, every drop gradually increasing the instability.
The longest-lived descendant of uranium 238 is uranium 234, a radioisotope with a half-life of 245,000 years; a long period of time that is still only one twenty-thousandth of the 4.5 billion year half-life of uranium 238. After a few half-lives of uranium 234 – about a million years later – the equilibrium we see today was established.
This secular equilibrium can occasionally be disrupted when one of the intermediary nuclei leaves the area where its ancestors are confined. These local disruptions are important to consider in the use of dating techniques.
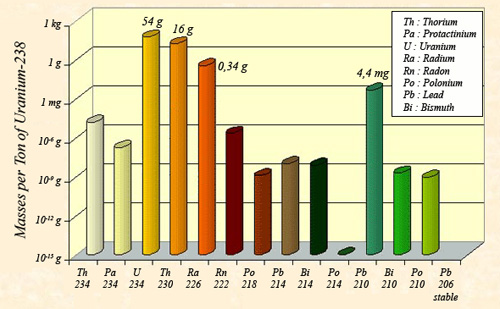
Masses of the descendants of uranium 238 at equilibrium
The law of radioactive equilibrium states that the activities of the descendants of uranium 238 must be equal to the activity of the ancestor nucleus: 12.4 million becquerels per tonne. One consequence of this equilibrium law is that the masses of the descendants present are proportional to the ratio that exists between their half-lives. These periods beeing very diverse and all much smaller than the period of uranium 238 (4.47 billion years), the masses are also far smaller: For a U-238 tonne, one finds 54 grams of U-234, 16g of thorium 230 and 0.34g of radium.
© IN2P3
A good example of equilibrium disruption is that of radon, one of uranium’s gaseous descendants that can escape from the minerals it gets trapped in. In the tunnel of uranium mines, fans can accelerate the diffusion of radon into the atmosphere. Considering the mine and the atmosphere as one system, the secular equilibrium is still maintained. In the mine, however, uranium’s descendants stop at radium – the parent of radon. The lineage continues outside of the mine, though the equilibrium has been disrupted in both areas.
Other articles on the subject « Radioactive decay law »
Radioactive Half-life
The half-life determines how quickly a radioisotope decays The ‘half-life’ of a radioactive nucle[...]
Radioactive Activity
The decay rate and the rate of radiation emitted The activity of a sample of radioactive matter i[...]
Radioactive Series
A long Radioactive Lineage A certain number of natural radioactive nuclei are still present on Ea[...]