A legacy of atmospheric nuclear bomb tests and accidents
Caesium 137 is a radioactive element with a relatively long radioactive half-life of 30.15 years. This particular isotope of caesium is both a beta and gamma emitter. It is produced in some abundance by fission reactions. A lot of attention is focused on caesium 137 : it is the main source of long term contaminations after atmosphric tests of atomic bombs and nuclear accidents. Along with strontium 90 and a handful of plutonium isotopes, it will also be the main source of radiation from radioactive waste for the next hundred or so years.
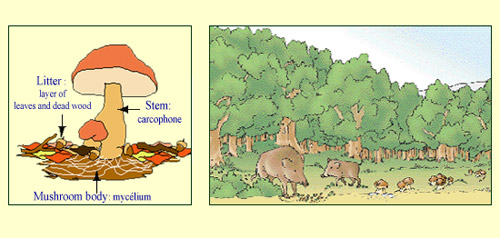
Caesium and the food chain:
Caesium 137 is the principal source of radioactive contamination in the food chain, mainly due to the nuclear bomb tests and the Chernobyl accident. Not a very mobile element, it slowly enters the soil, gradually reaching the leaves, then the roots. Today it is the mycelia of mushrooms and wild game that are the most contaminated. This contamination can vary from 15 to 5,000 becquerels per kilogram for mushrooms, and up to 5,000 Bq/kg for game. Nevertheless, to be exposed to a benign annual dose of 1 mSv, one would have to live in a forest and regularly consume the natural food one could find there.
© IRSN/dessin : Martine Beugin
Strontium 90 and caesium 137 are, in fact, the two principal fission products that have medium-length half-lives. About 10 kilograms of the former, and 24kg of the latter, are produced every year in a conventional pressurized water reactor (PWR)
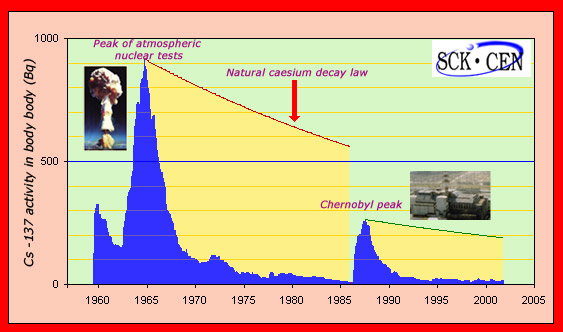
Progress of caesium 137 in the human body:
The activity of caesium 137 in the human body has been continuously monitored by the Mol laboratory in Northern Belgium for the past half century. Despite being far removed from the sites of nuclear tests and the Chernobyl accident, one can see very clearly the peaks in activity caused by the tests and, with a smaller magnitude, the peak of Chernobyl. In both cases, the decline of caesium 137 found in the body is much faster than the natural radioactive decline of a 30 years half-life. Even though caesium slowly enters the ground, the faster activity decline in humans has made it unnecessary for the Belgians to decontaminate their soil.
© SCK-CEN
The atmospheric nuclear bomb tests carried out in the 1960s contributed to spread caesium 137 on the Earth surface, which meant that for a while the isotope constituted between 1 and 4 % of the human body natural radioactivity. With the natural dispersion and decay that has occurred over the following decades, the amount of caesium that affects us has dramatically decreased
Caesium 137 is also one of the principal sources of radioactive contamination after nuclear reactor accidents. After Chernobyl, for instance, large quantities of caesium were released into the atmosphere, but by 1995 the levels of radiation had dropped below what they had been before the 1986 accident.
In the natural environment, caesium is far less mobile than iodine for instance. Attached to minerals, it tends to stay on the ground and is particularly abundant on the forest floor, after having been intercepted by the vegetation. The mycelium, the name given to the vegetative part of fungi and mushrooms that grow within a few centimeters of the ground, traps caesium in the same way as it (unintentionally) traps pesticides. The isotope can concentrate in the food chain: in the flesh of fish or game, for instance
Once absorbed by a human body, caesium spreads through the muscles until, after the 100 days of its biological lifespan, it disappears. This comparatively quick elimination means that only one nucleus out of 160 will decay inside the human body
In the world of industry, caesium 137 is used for its characteristic penetrative gamma ray signature
Spent reactor fuel contains other isotopes of caesium; particularly caesium 134, with a relatively short) half-life of two years and caesium 135 with a particularly long half-life of 2.3 million years.
Other articles on the subject « Main Radioactive Nuclei »
Uranium 238 and 235
A radioactive and strategic element The uranium atom is the heaviest atom present in the natural [...]
Plutonium 239
Plutonium 239: an artificial fissile nucleus, highly sought-after and feared Plutonium, the ninet[...]
Plutonium Properties
A transuranic element with long-lived radiotoxic isotopes Plutonium is a very dense metal, radioa[...]
Radium
The radioactive nucleus that made History Radium is an extremely rare element that was first disc[...]
Carbon-14
A by-product of cosmic rays Carbon-14 (C-14) formation in the atmosphere The nucleus of carbon-14[...]
Potassium-40
A curiosity of Nature and a very long lived beta emitter Potassium 40 is a radioisotope found in [...]
Iodine 131
Radioactive iodine : A dangerous and short lived fission product Iodine 131 is a radioisotope wit[...]
Tritium
A radioactive isotope of hydrogen Tritium is a beta-emitting radioactive isotope of hydrogen. Its[...]
Strontium-90
A fission product with properties close to calcium Strontium-90 is with cesium-137 a major radioa[...]
Technetium 99
A pure gamma emitter widely used in nuclear medicine Of all the atoms below uranium in Mendeleyev[...]