When a gamma expells an atomic electron and is absorbed
Internal conversion is a nucleus desexcitation mode which competes with gamma emission. It occurs after a beta or alpha radioactive decay has left the nucleus in an excited state. Internal emission can de viewed as the gamma emission where the gamma vanishes as it interacts with one of the atomic electron of the atom to which it transfers its energy. For that reason, it is also called electronic conversion.
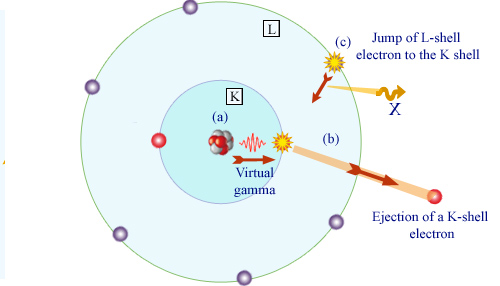
Principle of internal conversion
An excited nucleus emits a gamma ray (a). This gamma interacts with one of the inner electrons of the atom (b), most likely with an electron of the K layer. The electron is ejected from the atom. Absorbed by the electron, the gamma vanishes. The ejection creates a void in the layer where the electron was. The atom reorganizes : a more externel electron, here belonging to layer L, comes to occupy the void (c). An X-ray is emitted.
© IN2P3
The expelled electron inherits the gamma energy, but it must escape from the forces that bind it to the atom. Once free, its energy is dimininished of its former atomic binding energy. Remembering the shell structure of the atom, the binding energy is that of the layer to which the electron belonged. The energy transferred to the electron is that of the gamma diminished of the characteristic binding energy of the electron on its atomic layer. Both the gamma and the binding energy have well-defined values. Thus the energy of the expelled electron takes as well a series of characteristic values (one for each layer). The internal conversion probability is the largest for electrons belonging to the innermost K layer and decreases rapidly with the outermost layers.
Conversion electrons are characterized by a unique energy, as opposed to the electrons of beta decays whose energy energy vary between 0 and a maximum value, a part of the decay energy being carried by an invisible neutrino.
The electron expulsions are followed by a reorganization of the electron atomic cloud, with the emission of X-rays.
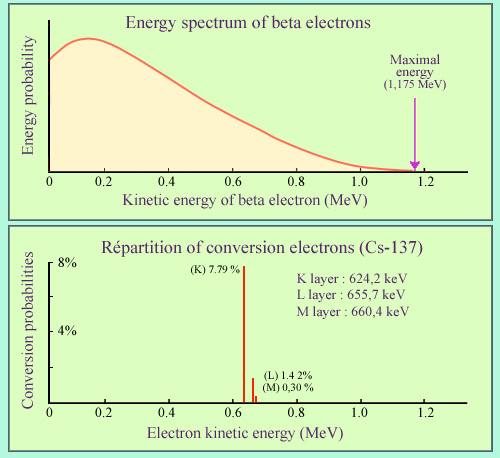
Beta electrons compared to conversion electrons
The example chosen is that of caesium-137, well known a beta-rays emitter. In 85.1% of cases the beta electron is accompanied by a 661.57 keV gamma ray and in 9.6% of cases by an electron conversion. While the energy distribution of beta electrons is continuous, the conversion electrons have a unique energy depending on the atomic layer from which they originate. The most common conversions occur in layer K. In the case of caesium-137, conversion electrons carries on average 5% of the decay energy.
© IN2P3
The example displayed of cesium-137 shows the respective weights of gamma emission and internal conversion. 94.7% of beta decays lead to an excited state of the nucleus : 85.1% return to the ground state by emitting an energetic 661.57 keV gamma, while 9.6% returns to stability through internal conversion. The electron conversion energy are a little smaller to that of the gamma.
In the general case, he existence and the energy of conversion electrons remain linked to the gamma rays emitted by the nucleus. Their contribution to the decay energy, which adds up to that of beta electrons, is at most of a few %.
Other articles on the subject « Alpha Beta Gamma rays »
Alpha (α) radioactivity
How heavy nuclei lose weight … by emitting alpha particles Alpha (α) radiation was first ob[...]
Beta (β) radioactivity
How Nature corrects an excess of protons or neutrons Beta (β) radioactivity was first observed in[...]
Beta spectrum
Beta electrons do not have a unique characteristic energy Three particles, the recoil nucleus, th[...]
Electron Capture
A minor mode … competing with positron emission Electron capture is a comparatively minor d[...]
Positron
The positive electron, the first ambassador of antimatter The positron was discovered in 1933 by [...]
The electron-neutrino
An electron that would have lost its electric charge The easiest way to conceive a neutrino is to[...]
Muons
An heavy electron abundant in cosmic rays Muons were first observed in 1936 by the Americans phys[...]
Radioactivity Gamma (γ)
How nuclei get rid of excess energy It was in 1900 that the French physicist Paul Villard first f[...]
Nuclear Desexcitations
Gamma are the light emitted by atomic nuclei A french proverb used to say « all roads go to Rome.[...]
Nuclear Transmutations
The ancient dream of the alchemists… The alchemists of the Middle Ages and the Renaissance [...]