How heavy nuclei lose weight … by emitting alpha particles
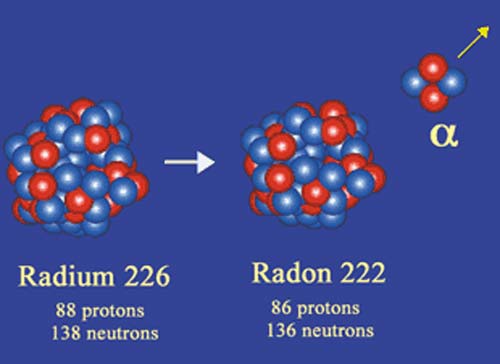
Example of the historical decay of radium-226
A radium nucleus is a massive nucleus of 226 nucleons, including 88 protons and 138 neutrons. It decays by emitting an alpha particle composed of two protons and two neutrons. The radium nucleus turns into radon-222 nucleus, itself radioactive, containing two protons and two neutrons less. The disintegration releases 4.6 million electronvolts of energy. The alpha particle carries the 222/226ths of this available energy and the radon 4/226ths. The process is very slow : on average, it is necessary to wait 2300 years to observe the disintegration.
© IN2P3
Alpha (α) radiation was first observed as an unknown type of ray that curved in the presence of electric and magnetic fields. The direction of curvature revealed that it had to be carried by particles carrying positive electrical charge, and in 1908 Ernest Rutherford was able to identify these ‘alpha particles’ as helium nuclei, with a resulting electric charge of +2e. Later it was found, after the neutron’s, discovery that the helium nucleus consists of 2 protons and 2 neutrons.
The emission of alpha particles is a property of the heaviest nuclei, such as uranium 238 with its 92 protons and 136 neutrons the heaviest natural nucleus observed.
These unstable nuclei emit a light helium nucleus in order to reduce their mass and hence increase their stability. It turns out that expelling two protons and two neutrons in this manner is more energy efficient than expelling the four particles individually.
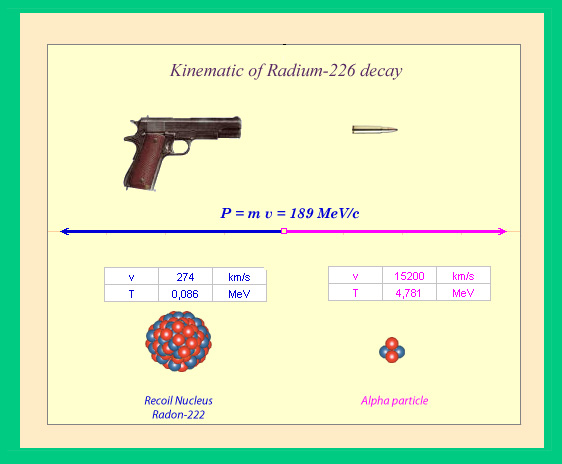
Analogy with the recoil experienced by a firearm:
The kinematic of an alpha decay are quite similar to those of a firearm where a light bullet takes away most of the energy of the explosion. The momenta (products of mass times velocity) of the recoiling nucleus and the alpha particle are exactly equal. The respective speeds and energies are inversely proportional to the masses, and as a result are highly unequal. In the case of a radium nucleus, the alpha particle takes away 98.3% of the available energy. These alpha particles are always emitted with the same energy values.
© IN2P3
The energy released in alpha decay takes the form of kinetic energy shared between the released alpha particle and the nucleus that expelled it. Much like in artillery fire, where the shell absorbs much of the energy of the explosion, the alpha particle takes away about 98% of the energy and the original nucleus (like the recoil of a cannon firing) gets the rest. The energy of the alpha particle is larger than for beta and gamma decay processes, and is usually of the order of four million electronvolts (MeV).
An example of alpha decay is the historically important transformation of radium 226 into radon 222 through the emission of an alpha particle. This reaction releases 4.6 MeV, and leaves behind a radioactive noble gas (radon), which is what allowed Rutherford to observe the process in Montreal in 1898.
The ‘half-lives’ of alpha disintegrations are often very long, and alpha emitters such as thorium 232 and uranium 238 can take billions of years to completely decay. Radium 226 decays with a half-life of 1600 years, so half of the radium nuclei present at the sacking of Rome have yet to decay – a nucleus which is less radioactive than commonly assumed.
Other articles on the subject « Alpha Beta Gamma rays »
Beta (β) radioactivity
How Nature corrects an excess of protons or neutrons Beta (β) radioactivity was first observed in[...]
Beta spectrum
Beta electrons do not have a unique characteristic energy Three particles, the recoil nucleus, th[...]
Electron Capture
A minor mode … competing with positron emission Electron capture is a comparatively minor d[...]
Positron
The positive electron, the first ambassador of antimatter The positron was discovered in 1933 by [...]
The electron-neutrino
An electron that would have lost its electric charge The easiest way to conceive a neutrino is to[...]
Muons
An heavy electron abundant in cosmic rays Muons were first observed in 1936 by the Americans phys[...]
Radioactivity Gamma (γ)
How nuclei get rid of excess energy It was in 1900 that the French physicist Paul Villard first f[...]
Nuclear Desexcitations
Gamma are the light emitted by atomic nuclei A french proverb used to say « all roads go to Rome.[...]
Internal Conversion
When a gamma expells an atomic electron and is absorbed Internal conversion is a nucleus desexcit[...]
Nuclear Transmutations
The ancient dream of the alchemists… The alchemists of the Middle Ages and the Renaissance [...]