Gamma are the light emitted by atomic nuclei
A french proverb used to say « all roads go to Rome. The proverb could be applied to a radioactive decay, considering that Rome is the outcome of transformations which lead to a nucleus, the final product of the disintegration. The radioactive nucleus follows a variety of roads to arrive to a common final state, a common terminus.
The state of a nucleus who has reached stability (or quasi-stability in the case of a radioactive filiation) is called by physicists its ground state. This is the one whose energy is minimal. Intermediate transitory states of the nucleus with a higher energy are excited states.
Radioactive decay rarely do not always leave a nucleus in its ground state. Often, the nucleus ends up in an excited state. In classical mechanics, the energy of the excited state could be any. But a nucleus, like the atom should obey the laws of quantum mechanics. The mechanics of the infinitely small say that a nucleus can exist only in a limited number of states characterized by an energy level, in the same way that atomic electrons belong to specific layers associated to a limited number of energy levels.
An excited state is associated with an unique characteristic value of the excitation energy. It corresponds to a nucleus state allowed by quantum mechanics that physicists casually refer as a « spin-parity » state. The nucleus spin value represents somehow the nucleus rotation whose protons and neutrons which behave like small spinning tops can have also a global rotation. The spin state is defined by an half-integer number (eg 1/2,3/2,5/2 …) when the number of nucleons is odd or by an integer number (for eg 0,1,2 …) when it is even. The parity state, a quantum property, is either positive or negative.
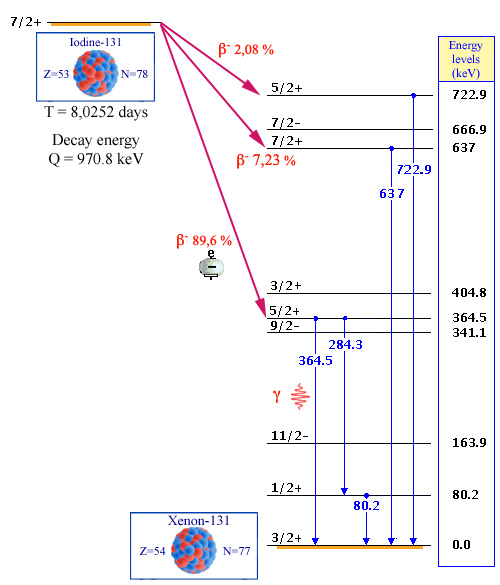
Example of the Iodine-131 decays
The energy available in this decay is important, 970,8 keV. Eight energy levels of the son-nucleus (xenon 131) are accessible. The three most frequent ways are represented by the red arrows. 89.6% of beta decays lead to the 364 keV energy level. The excited nucleus then emits either a 364 keV gamma gamma or two consecutive 284 and 80 keV ones. Other levels joined the ground state, either directly or by cascading to the ground state emitting one or more gamma rays. Only those with a frequency above 1% have been represented on the diagram.
© IN2P3
So in the case of iodine-131, the total decay energy is 970.8 keV; eight excited states of the nucleus can be reached. The iodine nucleus that emits a beta electron to become xenon-131 – the son-nucleus – has the choice between 8 excited states and the ground state. Energy levels can be viewed as the rods of a ladder. Having landed on one of thes rods, the nucleus tumbles from rod to rod to get to the bottom of the ladder. It may skip one or more energy levels to get faster to its terminus.
Jumping from one level to a lower level, the nucleus loses a part of its excitation energy by emitting a gamma ray whose energy is equal to the difference between the two levels. It may also, less often, get less excited by internal conversion.
The diagram of a radioactive decay with many alternatives to reach the ground state is complex. The presence of a gamma spectrum with characteristic energies is its unmistakable signature. With detectors able to measure accurately gamma energies, nuclear physicists can identify with this signature the presence of extremely small amount of atoms of a radioactive specy.
In most of the cases, one or two energy levels play a dominant role. The diagram becomes simpler. For example in the case of iodine-131, 89.6% beta of decays lead to the 365.4 keV energy level which de-energizes by emitting either a 365.4 keV gamma, or two successive gammas of 80 and 284.3 keV.
Apart rare exceptions, the lifetime of the excited states are extremely short, usually billionths of a second. When the nucleus emits a set of gammas to reach its ground state, these gamma emissions appear simultaneous.
Physicists call branching ratios, the probabilities of the various decay modes. (NB: Detailed data on all nuclei decays can be found on the data base nudat2 of the Brookhaven National : http://www.nndc.bnl.gov/nudat2/)
ALSO : Internal Conversion
Other articles on the subject « Alpha Beta Gamma rays »
Alpha (α) radioactivity
How heavy nuclei lose weight … by emitting alpha particles Alpha (α) radiation was first ob[...]
Beta (β) radioactivity
How Nature corrects an excess of protons or neutrons Beta (β) radioactivity was first observed in[...]
Beta spectrum
Beta electrons do not have a unique characteristic energy Three particles, the recoil nucleus, th[...]
Electron Capture
A minor mode … competing with positron emission Electron capture is a comparatively minor d[...]
Positron
The positive electron, the first ambassador of antimatter The positron was discovered in 1933 by [...]
The electron-neutrino
An electron that would have lost its electric charge The easiest way to conceive a neutrino is to[...]
Muons
An heavy electron abundant in cosmic rays Muons were first observed in 1936 by the Americans phys[...]
Radioactivity Gamma (γ)
How nuclei get rid of excess energy It was in 1900 that the French physicist Paul Villard first f[...]
Internal Conversion
When a gamma expells an atomic electron and is absorbed Internal conversion is a nucleus desexcit[...]
Nuclear Transmutations
The ancient dream of the alchemists… The alchemists of the Middle Ages and the Renaissance [...]