An atomic bulldozer, strongly ionizing along a very short path
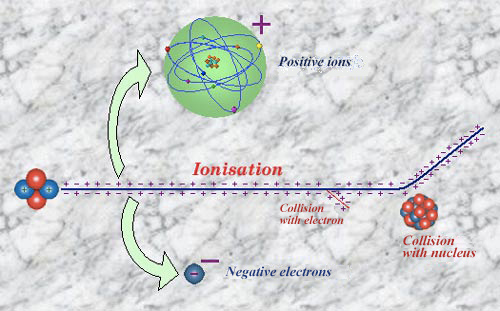
The wake of an alpha particle
An alpha particle is 7300 times heavier than an electron. It undergoes practically no change in direction when it ionises other atoms. It leaves behind it, strewn around its straight path, a wake of ejected electrons and ionised atoms. Occasionally, when an electron is released with a great speed, it may also in turn ionise. Alpha particles may sometimes collide with an atomic nucleus, resulting in a deflection at large angles. These very rare collisions, allowed Ernest Rutherford to deduce the existence of atomic nuclei.
© IN2P3
Alpha particles are simultaneously the most dangerous form of radiation for living matter and the easiest to protect oneself from. A sheet of paper or the thickness of a clothing item is more than enough to stop them and render them harmless.
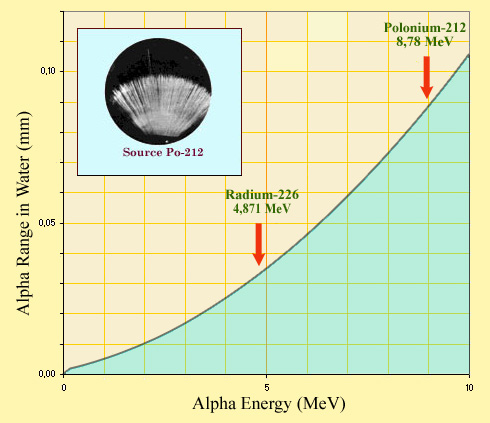
A very short trajectory !
Because of their great ability to ionise, the trajectory of alpha particles through matter is very short. In a dense medium such as water, even those energetic alpha particles emitted by polonium 212 can barely travel less than a tenth of a millimetre. The insert shows that inside a ‘cloud chamber’ saturated with vapor, the range of alpha particles does not exceed a few centimetres. It is very easy to protect against alphas from an external source.
© IN2P3
An alpha particle ejected from a nucleus is travelling at a speed greater than the fastest of man-made rockets. This speed, which can reach 9,600 kilometres per second for an alpha particle with 2 MeV of energy, is nonetheless much smaller than that of light – almost 300,000 kilometres per second. An alpha particle is also much heavier than the atomic electrons it bumps into, with a mass ratio of around 8000:1!
An alpha particle en route is like an atomic bulldozer, unperturbed by the electrons it rips away from the atoms met along its travel path. Travelling in a straight line, and at a comparatively low velocity (compared to electrons), it can force large numbers of atoms to give up their electrons in a process known as ionisation .
.
The alpha decays caused by the trace amounts of uranium found in blocks of granite will never escape the confines of the rock. Those particles emitted by atoms at the surface, however, will be stopped by the thickness of a sheet of paper, a pair of trousers or a glove.
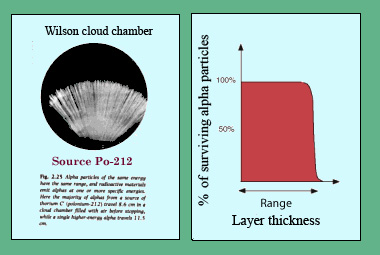
A well defined range
This historic photograph shows the trajectories of 9 MeV alpha emitted from a radioactive source of thorium-C (polonium-212) inside a Wilson cloud chamber. The ‘shaving-brush’ aspect is due to the fact that alpha, issued with the same energy, have a well defined range. They travel no farther than 11.5 cm in the chamber. The curve on the right illustrates the same effect : the percentage of alpha particles crossing a given thickness of matter, remains at 100% before falling to 0 when this range is reached.
© IN2P3
The only time an alpha particle is dangerous is if it attaches itself inside living matter. Contact with the skin is not enough, as the epidermis is thick enough for the radiation to be absorbed entirely within the ‘dead’ layers of skin. Real risks arise from the inhalation of radioactive aerosols which come to rest on the cells of the lungs. When an alpha emitter finds itself inside an organ it can cause considerable damage, as the living cells absorb the brunt of the energy over very short distances.
Alpha particles, which carry a positive electric charge of +2, are repulsed by the nuclei of other atoms. They do not have in general enough energy to enter in contact with target nuclei and induce nuclear reactions. There are exceptions : for instance in order to obtain neutrons sources, beryllium or boron nuclei (which are light nuclei) are bombarded by the alpha particles of americium-241 sources.
In the general case, alpha particles can approach target nuclei and bounce back at large angles. These rare collisions have played an important part in the history of nuclear physics. It was by observing alpha particles travelling through a thin sheet of gold, and noting that they occasionally collided at large angles with unseen bodies, that between 1909 and 1911 Geiger, Marsden and Rutherford were able to deduce the existence of atomic nuclei.
Access to page in french
Other articles on the subject « Radiations effects in matter »
Charged Particle Effects
A gradual loss and transfer of energy Alpha rays, fission products ; heavy, slow and ionizing par[...]
Beta Rays in Matter
Light electrons : a chaotic journey through matter Beta electrons and positrons have equal and op[...]
Bremsstrahlung
A relativistic phenomenon that applies to electrons and positrons… The phenomenon of bremss[...]
Neutral Particle Effects
Energy transfer by proxy… Neutral particles that are of interest in the field of radioactiv[...]
Cherenkov Effect
When an electron goes faster than light in air and water … The Cherenkov effect occurs when[...]
Cross Section
Cross section or the interaction probability of a particle Cross section is the name given by phy[...]
Gamma Rays in Matter
Gamma can be attenuated but never fully stopped The neutral gamma rays leave very different effec[...]
Photoelectric Effect
The most effective mechanism of photon absorption The photoelectric effect is the phenomenon that[...]
Compton Effect
Photons as projectiles and electrons as targets The Compton effect is the name given by physicist[...]
Macroscopic Effects
Effects on inert or organic matter The ionisation of atoms surrounding the trajectory of an alpha[...]