Particle and wave: an effect of quantum mechanics
The great age of uranium and thorium nuclei that reach billions of years shows that the alpha decay occurs with difficulty, although they release millions of electron volts of energy .
These nuclei would be perfectly stable without a laborious process that overcomes the nuclear forces and triggers decay. The attractive effect of the nuclear force stops abruptly out of the nucleus. If four nucleons, grouped into one alpha particle, are not anymore in touch with the other nucleons, this group feels only the repulsion due to the electric charge of the rest of the nucleus. Then it moves away faster and faster to acquire the kinetic energy of a few million electron volts which was discussed. The thing is to get to lose that contact.
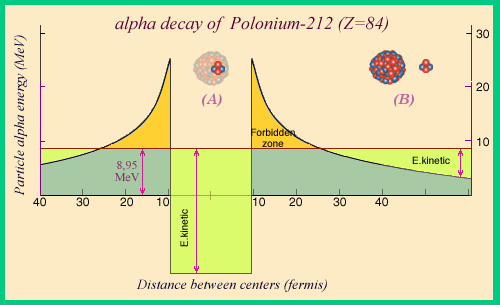
A potential well
The alpha decay of polonium-212 releases the most important quantity of energy, 8.95 MeV. This decay would be forbidden by classical mechanics : it would be impossible for an alpha particle to go from inside the nucleus in A to outside in B. It finds itself trapped at the bottom of a « well » as shown by the curve that represents the potential energy of interaction between the particle and the rest of the nucleus. To get from A to B, the particle must pass through a restricted area where its kinetic energy would be negative. The permitted areas are the well where nuclear attraction dominates, and outside the well where the repulsion due to the nuclear charge prevails.
© IN2P3
We owe to a Russian-born American physicist George Gamow, the first explanation of alpha decay, a decay that is not authorized by the laws of classical physics.
The mechanism proposed by Gamow was called « quantum tunneling » . The quantum tunneling or “tunnel effect” describes the fact that a particle behaves as both a particle and a wave in the infinitesimally small world where quantum mechanics replaces classical mechanics.
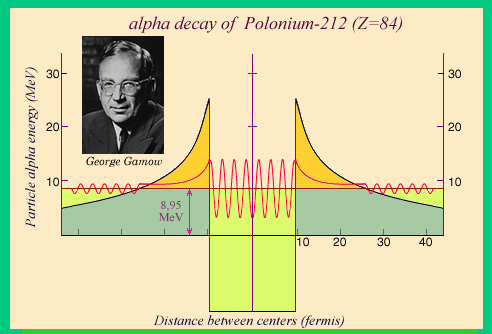
The Tunnel Effect
The wave associated with an alpha particle trapped inside a nucleus has been superimposed to the previous figure. We see that the wave extends slightly outside the nucleus, where the oscillation amplitude has been amplified to make them visible. The square of the amplitude of the oscillations is, in quantum mechanics, the probability of observing the particle at a given position. So there is a probability of observing the alpha particle outside the nucleus, that is to say a decay.
© IN2P3
The alpha particle is found in the situation of a mountain climber, trapped in a crater, which has no more strength to join the mountaintop, passes on the other side and slide down toward the valley. The barrier to cross reflect the competition between the attractive nuclear force and the electrostatic repulsion. The alpha particle can not cross the barrier because it does not have the necessary energy: it is either inside or outside the nucleus. At least for classical mechanics.
In quantum mechanics, the situation is less obvious. The wave, which represent an alpha particle in the nucleus, is not strictly localized and slightly overlaps the other side of the fence. There is a probability of observing the particle outside the nucleus, where the nuclear force is no longer felt. This probability is extremely small, but it enables the decay. Again with the image of the climber, the trick at his disposal to win the other side of the mountain and find freedom, is to dig a tunnel through it.
An empirical law says that higher is the potential barrier, larger is the thickness to cross and greater is the nucleus lifetime. This explains some particularly long lifetimes.
Related topic : Beta decay : weak forces
Other articles on the subject « Radioactive nuclei »
Map of Nuclei
Map of stable and unstable nuclei The progress made in our understanding of the subatomic world o[...]
Stability Valley
Beta decay : nuclei getting slimmer to achieve stability The nucleus mass is related to its inter[...]
Nuclear Forces
Three nuclear forces and their hierarchy Three types of force act alongside each other inside a n[...]
Mecanisms of Radioactivity
The way radioactivity works and its origins The impressive range of half-lives that exist, extend[...]
Alphas with gammas
Gamma rarely accompanies alpha decays It is surprising that one can hold with hands a sample of p[...]
β decay : weak forces
The forces which allow a nucleus to emit beta electrons Beta decay (β) and electronic capture cha[...]
Weak Forces
A special and fascinating fundamental interaction A third force is at work in the nucleus next to[...]
The Nucleosynthesis
Primordial and stellar nucleosynthesis The Universe hasn’t always existed, It was born a li[...]
Nucleosynthesis (continued)
Mechanisms of atomic nuclei formation Most of the nuclei of atoms that make up our daily life wer[...]