The forces which allow a nucleus to emit beta electrons
Beta decay (β) and electronic capture change the composition of protons and neutrons in a nucleus, the electric charge of the nucleus increasing or decreasing by one. This variation of charge is compensated by the emission of a charged particle – an electron or a positron – or, more rarely, by the capture of an electron. As another characteristic signature of these transformations, other particles that cannot be detected are emitted: neutrinos or antineutrinos.
The main forces at work in the nucleus, those attractive that maintain its cohesion and those repulsive between electric charges of the same sign are unable to transform neutrons into protons and produce electrons, positrons, neutrinos and antineutrinos. Nature therefore uses a third type of interaction (this term is somehow more accurate than force) to allow and proceed beta decay or electron capture.
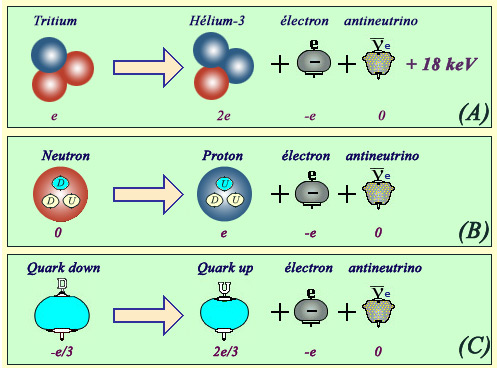
From quarks to nucleus
The beta decay of nuclei are the result of changes that occur at more elementary levels. For example, the transformation of a tritium nucleus (A) to helium-3 is caused by the transformation of one of the two tritium neutrons into a proton. The transformation itself of a neutron into a proton (B) is the result of a transformation of its elementary constituents : quarks (Neutrons composed of 2 down quarks and 1 up quark, protons of 2 up and 1 down quars ). It is the transformation of a down quark into an up quark (C) that is the origin of the mutation of a neutron into a proton, thus the beta decay of tritium.
© IN2P3
This third interaction is considered weak because beta decays that are the most visible manifestation are very slow transformations that happen rarely. The lifetimes of unstable nuclei are extremely variable (quarter of an hour for a free neutron, one week for iodine-131, thirty years for cesium-137, a billion years for potassium-40), but all these periods, including the quarter of an hour of the neutron, are very long for the nuclear clocks.
The first theory of beta decay was made in 1934 by the great Italian physicist Enrico Fermi, at a time when the existence of quarks was not suspected and the one of neutrinos only hypothetical. Since the 1970s, we know that when a nucleon changes its nature (proton or neutron), it is because one of the constituents (up or down quark) transforms itself from one species into another. It is at this elementary level that weak interaction steps in.
In the case of beta-minus decay mechanism is as follows. A down quark in a neutron, whose electric charge is -e/3, frequently emits a negative charge -e. Its charge is now +2e/3. It has become an up quark. In general, the up quark reabsorb immediately the negative charge and returns to the down quark state. The negative charge briefly emitted and immediately reabsorbed is carried by an unstable particle called the W-minus boson.
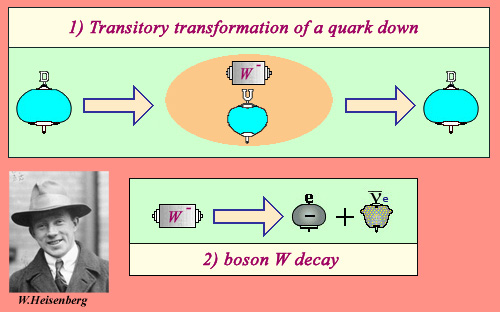
Heisenberg « uncertainty principle »
We owe to the great German physicist Werner Heisenberg (1901-1976) to have formulate the uncertainty principle of quantum physics in 1927. He was only 26 years! This principle describes how a particle can change its nature during a very short time during which its energy can not be definite with precision. In the case of a down quark transformation into an up quark with a W boson, the uncertainty principle makes it possible to quantify this time to 10-26 second. Normally the W boson is reabsorbed by the quark which becomes again « down ». However during this extraordinarily short time, there is a very small probability for the W boson to decay into an electron and an antineutrino. This is the origin of beta radioactivity.
© IN2P3
If the boson decays in the extraordinarily short time elapsing between its emission and its reabsorption, a beta-minus decay occurred. This electron-neutrino W decay mode, the most economical in energy occurs in the phenomena of radioactivity.
This mechanism is explained in the framework of quantum mechanics. It is the Heisenberg uncertainty principle that allows a quark to emit and reabsorb an object much more massive than him, the W boson. The existence of this fugitive intermediate, whose properties had been predicted by theory in the late 1960s, has been confirmed experimentally in 1983.
Related topic : α decay : tunnel effect
Other articles on the subject « Radioactive nuclei »
Map of Nuclei
Map of stable and unstable nuclei The progress made in our understanding of the subatomic world o[...]
Stability Valley
Beta decay : nuclei getting slimmer to achieve stability The nucleus mass is related to its inter[...]
Nuclear Forces
Three nuclear forces and their hierarchy Three types of force act alongside each other inside a n[...]
Mecanisms of Radioactivity
The way radioactivity works and its origins The impressive range of half-lives that exist, extend[...]
α decay : tunnel effect
Particle and wave: an effect of quantum mechanics The great age of uranium and thorium nuclei tha[...]
Alphas with gammas
Gamma rarely accompanies alpha decays It is surprising that one can hold with hands a sample of p[...]
Weak Forces
A special and fascinating fundamental interaction A third force is at work in the nucleus next to[...]
The Nucleosynthesis
Primordial and stellar nucleosynthesis The Universe hasn’t always existed, It was born a li[...]
Nucleosynthesis (continued)
Mechanisms of atomic nuclei formation Most of the nuclei of atoms that make up our daily life wer[...]