The elementary components of light and electromagnetic waves
Light is composed of infinitesimal individual electromagnetic waves known as photons. Each photon carries a minuscule amount of energy. Max Planck and Albert Einstein referred them as a ‘quantum’ of energy when they first established the particle nature of light.
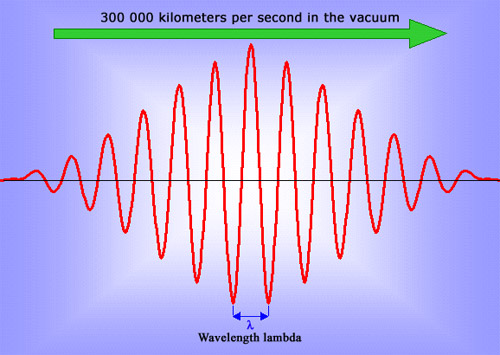
The photon : an elementary fundamental wave
A photon is a microscopic electromagnetic wave, caused by oscillations in the electric and magnetic fields through space and time. A wave, in the classical sense of the term, consists of infinite oscillations and can never be localised. The number of oscillations in the photons emitted by atoms and nuclei is not infinite, thanks to a small uncertainty in their energy values. The shorter the wavelength of the photons, the denser the wave is in space. When the wavelength is as short as is the case with gamma rays, then the waves become dense enough to resemble particles.
IN2P3
Despite what the Greek name would suggest, photons do not carry always light. Light waves do have the remarkable property of leaving an impression on our retinas, but many other photons exist that we do not see.
One groups together under the name ‘photon’ a variety of electromagnetic rays, from radio waves to X Rays and gamma rays, in addition to infrared, visible and ultraviolet light.
Like all waves, light can propagate. The speed of light – and more generally, the speed of electromagnetic waves – in a vacuum is the largest speed known and a universal speed limit: it is impossible to travel faster. It is equally impossible to slow a photon down, a fact which physicists have interpreted as indicating that photons have no mass.
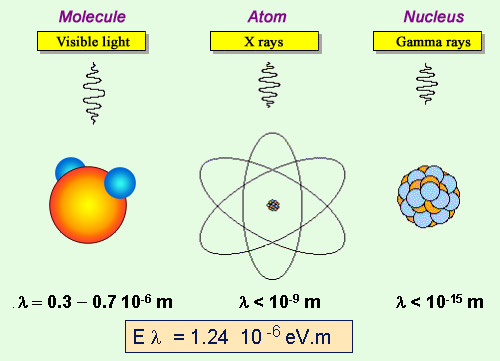
Three types of photons
The photon energy varies inversely with its wavelength. Photons are usually emitted by molecules, with energies of a few electronvolts and wavelengths of a fraction of a micron (a thousandth of millimeter). X-rays are emitted by the deeper layers of the atom, with energies reaching hundreds of thousands of electronvolts (keV) and wavelengths of the order of a billionth of a meter (nanometers). Gamma rays, on the other hand, are emitted by nuclei and have even higher energies with wavelengths appropriate to the dimensions of a nucleus.
© IN2P3
The impossibility of travelling faster than the speed of light in vacuum (one of the fundamental constants in physics) is at the core of Einstein’s theory of relativity.The relativity of time has no effect on our daily lives. In nuclear and particle physics, however, where scientists study particles whose speed approaches the speed of light, relativistic effects are very common.
As quanta of electromagnetic energy, photons are defined by … their energy. As a fundamental wave, they are also defined by their wavelength. Albert Einstein was the first to show that the product of a photon’s energy by its wavelength is a constant – 1.24 electronvolt microns (if one chooses units of energy and length appropriate to photons of light).
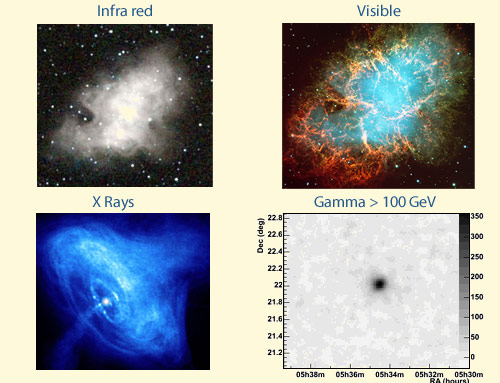
From infrared to gamma rays
This diagram shows the same celestial object – the Crab nebula – as seen by different types of photons: infrared, visible light, X-rays and gamma rays. All these photons have the same nature, though their wavelengths (which define the colour in the visible part of the spectrum) and energies are different. The image taken with high-energy gamma rays was obtained thanks to the 4-telescope setup of the HESS experiment, used to identify and measure the direction of high-energy gamma rays.
© IN2P3/LPNHE
According to this equation, photons with higher energies have shorter wavelengths and vice versa. Photons emitted by nuclei have an energy about a million times higher than that of photons emitted by a molecule or an atom, and were first discovered by Paul Villard in 1899. The tiny wavelengths of these ‘gamma rays’ makes them very compact and capable of easily passing through atoms, and as a result are highly penetrative.
Gamma rays emitted by nuclei have energies of the order of a million electronvolts (MeV). Particle physics focuses on gamma rays with much higher energies; of the order of several thousand MeV, if not higher.
Other articles on the subject « The atomic world »
The Atom
An almost empty space with mass concentrated in a tiny nucleus The atom is often viewed as a mini[...]
The electron
The best known of elementary particles The electron is an elementary particle that plays a fundam[...]
Atomic Energy Levels
A shell structure …. The conquest of space has familiarized us to the concept of a satellit[...]
Orders of Magnitude
The very small and the very large … The atom, and the nucleus in particular, belong to the [...]
Avogadro’s Number
Trillions of billions of very small atoms …. The microscopic size of atoms comes with their[...]
E=MC²
The Einstein formula, a relation between mass and energy The energy released by a chemical reacti[...]