Analogies with the atomic energy levels …
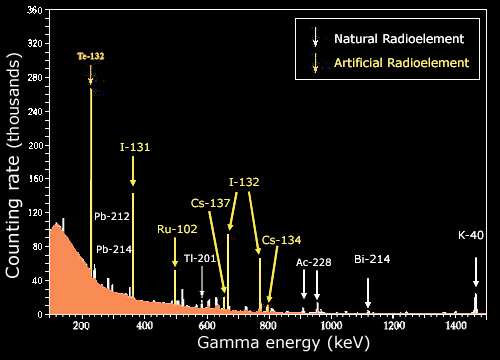
Gamma spectrum
A consequence of the nuclear energy levels is the emission of gamma photons with characteristic energies. These rays are frequently used to identify radioactive nuclei in a given sample. The image above shows the photons coming out of such a sample, and the precisely measured energies associated with each. One observes series of characteristic lines, indicating the existence of certain radioelements. The continuous background is caused by the presence of poorly detected gamma rays.
© IN2P3
At first glance, nuclei seem to be very different from atoms. More than a hundred thousand times smaller, they are also vastly more complex; atoms are primarily made up of empty space whereas the inside of the nucleus is extremely dense. Despite these differences, however, atoms and nuclei have a lot in common.
The behaviour of a nucleus is governed by the laws of quantum mechanics; laws which supersede those of classical mechanics at the microscopic scale. These quantum laws force the nucleus to exist in any one of a finite number of ‘states’, primarily defined in terms of the energy it possesses. This energy is at a minimum when the nucleus is in isolation – a state usually referred to as the ‘ground state’.
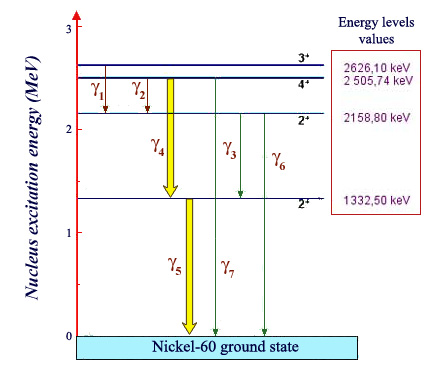
Energy levels of Nickel 60:
Energy ladder of a nickel 60 nucleus (product of the decay of the cobalt-60), indicating the energy levels the nucleus can reach. The lowest called the ground state, represents the energy of a nucleus at rest. If raised to one of the high levels, the nucleus immediately emits one or more characteristic gamma rays, lowering its energy sufficiently to allow it to reach the ground state. These energies are calculated by taking the difference between the departure and arrival energy levels. The transitions numbered 4 and 5, by far the more common, have been represented with thicker arrows.
© IN2P3
When the nucleus is at a different level, it finds itself with a surplus of energy. This extra energy is released in the form of a gamma photon, thereby allowing the nucleus to reach its ground state once more. These gamma photons are of the same kind as the X rays emitted by atoms, but have far greater energies – which can be of the order of a million electronvolts (MeV).
The energy states of the community of nucleons coexisting in any given nucleus can vary, primarily due to a kind of ‘layer’ structure not dissimilar to that which exists in the atom. The binding energy holding the nucleons together can take any of a selection of values that correspond to the number of ‘layers’ that exist.
Nuclei with 2, 8, 20, 28, 50, 82 or 126 nucleons are observed to be particularly stable; an interesting analogy with the stability of atoms of the noble gases whose outermost electron layers are said to be complete.
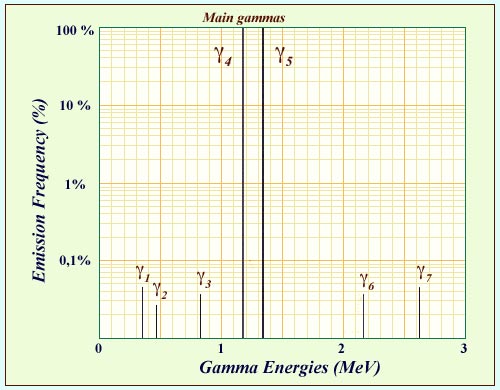
Cobalt-60 gamma spectrum
The excited states of nickel 60 are reached when cobalt 60, an isotope used in medicine, undergoes beta decay. On the nucleus’s path to the ground state, it emits a number of gamma rays. The diagram above shows the energies and the frequencies of these gamma. Two of these gamma rays are emitted practically 100% of the time. These two main gamma rays emitted in cascade have characteristic energies around 1 MeV (1.17324 and 1.33250 MeV). Their detection serves as an extraordinarily sensitive indicator of cobalt 60 decay.
© IN2P3
In addition to this layered structure, the nucleons can undergo collective movements which correspond to new nuclear energy states. If the entire cluster starts vibrating, for instance, the vibrational energies can only take certain specific values, precisely determined by the laws of quantum mechanics.
Finally, it is important to remember that these nuclei are not necessarily spherical in shape, and can undergo deformation or start rotating. The energies associated with these rotations can also only take well-defined values – they are said to be ‘quantified’.
Other articles on the subject « The atomic nucleus »
The proton
The charged constituent of the nucleus The nucleus of the hydrogen atom consists of one solitary [...]
The Neutron
The neutral partner of the proton The neutron is, along with the proton, one of the two constitue[...]
Isotopes
Nuclear variants of a given atom … Free of all electric charge and present only in the nucleus, n[...]
Quarks and leptons
The fundamental constituents of matter On the most elementary scale, the world around us is made [...]
Quarks and Gluons
How quarks interact within nuclei … To describe radioactivity and nuclear reactions such as[...]
Matter and Antimatter
Nature loves symmetry. However, one can not dream of a matter less symmetrical than the matter wh[...]