A new vision of the atom
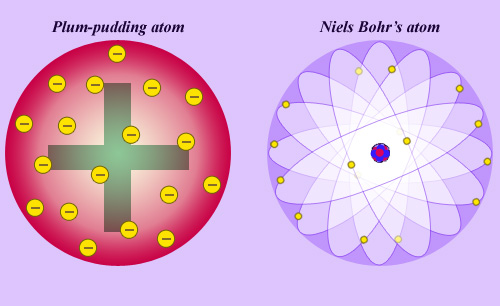
The plum-pudding atom, precursor of the modern atom
Before the discovery of the atom nucleus by Rutherford, a popular representation was that of a plum-pudding atom. Electrons carrying negative electric charges had been discovered in 1896, and it was speculated that these charged particles were moving inside a kind of magma of positive charges whose nature and layout were ignored. No one was imagining the modern atom proposed by Niels Bohr in 1913, essentially made of vacuum with all positive charges concentrated within a tiny nucleus.
© IN2P3
In 1911, Rutherford, Marsden and Geiger discovered the dense atomic nucleus by bombarding a thin gold sheet with the alpha particles emitted by radium. Rutherford and his students then counted the number of sparks produced by these alpha particles on a zinc sulphate screen. From this observation, they concluded that almost all the atomic matter was concentrated in a tiny volume situated at the atom center, the atomic nucleus.
The discovery of the nucleus led Niels Bohr to make the first theoretical representation of the atom. The ‘quantum mechanical’ revolution, culminating in the later developments made by Erwin Schrodinger, laid the foundations for our understanding of the infinitely small.
The study of the chemical properties of the elements in the uranium-radium family showed that radium D, radium B and radium G have the same chemical properties as lead. These elements, now known to be lead isotopes, took their names (which have since been forgotten) from their position in the radioactive lineage of radium which in the 1910’s had only recently been isolated and was used as radioactive sources for experiments. In 1913, Frederick Soddy concluding that these three elements belong to the same square of Mendeleyev’s periodic table, introduced the concept of ‘isotopes’, and later won the Nobel Prize for Chemistry in 1921.
This video takes us in 1911, in Manchester, on a journey through the history of particle physics., when Ernest Rutherford conducted a historical experiment that revealed that most of the mass of an atom is concentrated in a tiny nucleus made of protons and neutrons.
Alpha particles were the only nuclear projectiles available for physicists to use. Armed with radioactive sources, physicists were unwittingly examining the nuclei of atoms; so that by 1919 Rutherford was able to generate the first atomic transformation: a nuclear reaction. By firing alpha particles at nitrogen atoms, he was able to transform them into oxygen.
Over the following years, a large variety of nuclear reactions were observed and studied. The most important of these was the one which led to the 1932 observation of neutral radiation composed of particles with a mass approaching that of the proton. This was James Chadwick’s (1891-1973) discovery of the neutron. Soon after, Werner Heisenberg was the first to propose the theory that the nucleus was made up of protons and neutrons.
Other articles on the subject « Discoveries »
Marie Curie
Marie Curie Marie Sklodowska-Curie had an exceptional destiny. Born in Poland, she came to gradua[...]
Three radiations
Understanding the nature of alpha, beta and gamma rays In the years following the discovery of ra[...]
Ernest Rutherford
In October 1895, landed in England a 24 years-old young New Zealander. His name was Ernest Ruther[...]
Rutherford’s experiment
The experiment which proved the existence of a nucleus in the atom In 1908, Ernest Rutherford rec[...]
The neutron : Chadwick
A close competition between great physicists … James Chadwick, who discovered the neutron i[...]
Radium and Medicine
A short history: first steps in nuclear medicine … Everyone knows that the discovery of rad[...]
1934 : Artificial Radioactivity
The production at will of radioactive isotopes In the first days of 1934, Frederic and Irene Joli[...]
The Neutrino Hypothesis
The remarkable story of the neutrino The study of radioactive disintegrations had established tha[...]
Fission discovery
A nuclear phenomenon that escaped the hands of physicists In the years spanning 1934 to 1938, Enr[...]
Enrico Fermi
A genial experimenter and theoretician Enrico Fermi (Rome, September 29, 1901 – Chicago, No[...]