The neutral partner of the proton
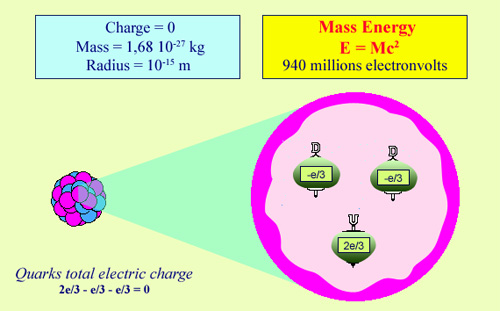
The structure of the neutron:
The neutron is one of the two constituent of an atomic nucleus. The neutron can be viewed as a proton that has lost its electrical charge. It is also a very small particle whose radius is equivalent to one millionth of a billionth of a meter, composed of even smaller particles known as quarks. As a particle, the neutron is unstable but once inside the nucleus it regains stability. Its lack of electrical charge allows it to be captured easily by nuclei and be the source of nuclear reactions.
© IN2P3
The neutron is, along with the proton, one of the two constituents of the atomic nucleus. The roles both particles play within the nucleus are so symmetrical that physicists have the tendency to treat them as two different states of one particle: the nucleon. The neutron can be seen as a proton with no electric charge; a difference that brings with it important consequences.
The attractive ‘strong’ force that holds the nucleus together affects both protons and neutrons in the same way. Gluing them, it is able to overcome the electric repulsion that the positively-charged protons exert on each other. In order to effectively counterbalance the increased electric repulsion felt by the protons in the heavier nuclei, these nuclei have neutron : proton ratios higher than one.
Neutrons are stable inside a nucleus. This structural stability is lost when neutrons are in a free, independent state. As the neutron is a little heavier than the proton, Einstein’s famous mass-energy relation equates this extra mass with an extra energy. This energy is just enough for the neutron to transform into a proton by emitting an electron and an antineutrino.
This transformation can also take place within a nucleus when there are too many neutrons present: the resulting electron emission is what is referred to as beta decay.
The average lifespan of a neutron in its free state is of the order of a quarter of an hour, which is why the only free neutrons we come across are those that have recently been formed. These neutrons are either produced by cosmic rays in the outer atmosphere, or freshly generated by our nuclear reactors.
The ideal agent for nuclear reactions
The neutron having, as its name suggests, no electrical charge, is not affected by the electrical charge on nuclei. It is thus an ideal projectile in the starting of nuclear reactions: as was noticed by Rutherford when he proposed his 1920 hypothesis of ‘a sort of neutral atom that was not of hydrogen’. The sudden intrusion of a neutron can disrupt the delicate equilibrium of a nucleus, sparking off a variety of nuclear reactions: the most famous being the uranium fission reaction.
Neutrons are released in large quantities in the core of nuclear reactors, following a series of fission reactions. The science of the neutron flux is known as ‘neutronics’, and mastery of the physics involved is essential to ensure the smooth working of reactors.
We also use neutron sources to produce radioactive atoms for medical, industrial and laboratory uses. The fact that neutrons are neutral means that there is no way to accelerate them through electric or magnetic fields. As a result, there are no neutron accelerators.
Learn more :
Neutrons Moderators
Other articles on the subject « The atomic nucleus »
The proton
The charged constituent of the nucleus The nucleus of the hydrogen atom consists of one solitary [...]
Isotopes
Nuclear variants of a given atom … Free of all electric charge and present only in the nucleus, n[...]
Nucleus Energy Levels
Analogies with the atomic energy levels … At first glance, nuclei seem to be very different[...]
Quarks and leptons
The fundamental constituents of matter On the most elementary scale, the world around us is made [...]
Quarks and Gluons
How quarks interact within nuclei … To describe radioactivity and nuclear reactions such as[...]
Matter and Antimatter
Nature loves symmetry. However, one can not dream of a matter less symmetrical than the matter wh[...]