A radioactive isotope of hydrogen
A low-energy beta electron
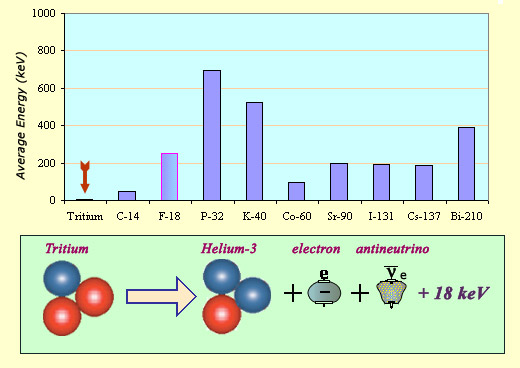
The energy carried away by a tritium electron is exceptionally low as can be seen from this comparison of average energies from a variety of beta decays: 5.7 keV when compared to several hundred keV for the others. The total energy liberated, shared between the electron and the antineutrino, is 18 keV. As the decay directly produces a ground state helium nucleus, there is no excited state and hence no gamma emission.
© IN2P3
Tritium is a beta-emitting radioactive isotope of hydrogen. Its nucleus consists of one proton and two neutrons, making it three times as heavy as a hydrogen nucleus (with its one proton) and 1,5 time as heavy as deuterium (which contains one proton and only one neutron).
Tritium would no longer exist in our environment if cosmic radiation produces it in the atmosphere in very small amounts. The half-life of the unstable tritium nucleus is of 12.3 years, which is very short on the radioactive time-scale. This comparatively fast disappearance means that very little tritium can accumulate in any place.

Luminous dials
Tritium has replaced radium in the luminous paint used in the dials of watches and navigational instruments. Today, the luminescent letters contain tritium as well as fluorescent substances which glow under the beta radiation emitted by the tritium. The manufacture, as well as the use, poses no problems to health. The low energy beta electrons do not leave the paint and no gamma radiation is emitted.
© Musée Curie
Its short half-life has also led to its classification as a highly radioactive element. This high activity, fortunately, is attenuated by some of the other properties of the decay processes. The average energy of the emitted electron tends to be very low – 5.7 keV as opposed to the several hundred keV in most beta decays. In addition to the low energy electron, tritium emits no gamma ray.
Tritium, like hydrogen, is particularly mobile. It can combine with oxygen to form tritiated water and therefore has the ability to get easily into the human body thanks to the water cycle. Once inside the body, tritium can lead to internal exposure though the element is eliminated very quickly. Its biological half-life of 10 days is far shorter than the 12.3 years of its radioactive half-life. Only one tritium nucleus out of 650 will decay while still inside the human body. Because of the low emission energy, the beta electron trajectory will not exceed a few microns inside the body.
An unusual fission reaction
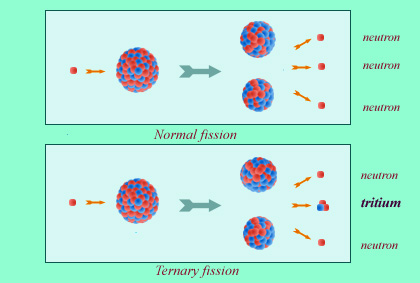
Tritium is found among radioactive waste products in reprocessing plants and military facilities. This tritium is produced by rare fission reactions – ternary fissions – in the nuclear fuel of reactors. It can also be produced in the primary water by neutron capture in lithium-based products.
© IN2P3
Tritium therefore has a particularly low radiotoxicity (dose factor). The World Health Organization, WHO) considers that the limit of acceptability of water containing tritium is 10,000 becquerels per liter. This limit is protective. One should drink 2 liters of such water everyday a day for a year to be exposed to a dose of 0.1 mSv per year equivalent to two weeks of natural radioactivity in France.
In February 2016, during the unloading operation of nuclear fuel unloading, tritium contaminated water above normal levels spread under the Indian Point nuclear power plant near New York. According to the Nuclear Regularity Commission – the US safety authority – this leak did not pose a threat to the environment. Indeed, tritium the tritium radoactive toxicity particularly little small. Once spilled into the Hudson, the radioactive water was so diluted that tritium became virtually undetectable. The same goes for tritiated effluents from the La Hague plant in France.
In biology, tritium is often used to mark hydrogen and thus in the study of metabolism. This has allowed us to narrow down the length of the biological half-life inside the human body to within 6 and 9 days.
In daily life, tritium has replaced radium in making dials of watches and navigational instruments luminescent.
Tritium can be found among the radioactive waste generated by reprocessing plants and military facilities, as they can be produced by ternary fission reactions (which are comparatively rare) occuring in the nuclear fuel of reactor cores.
Fusion of deuterium and tritium
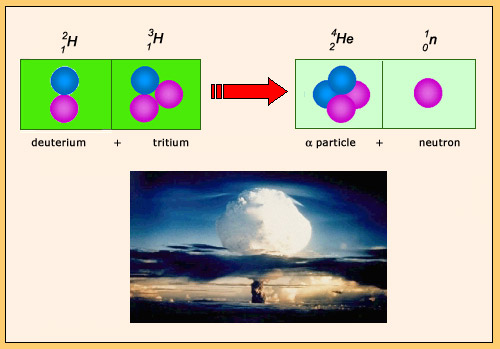
The fusion reaction of deuterium and tritium is the fusion reaction that liberates the most energy: 17 MeV. During the reaction, the nucleons reorganize themselves to produce an alpha particle and a neutron. The formation of the alpha particle is responsible for the large amount of energy released. This particular reaction is used both in H bombs and in laboratories to produce energetic neutrons.
© IN2P3
The fusion reaction of deuterium and tritium is the thermonuclear reaction which releases the most energy. This reaction was used by the United States and the Soviet Union in the 1950s and 1960s to test thermonuclear bombs or H-bombs, much more powerful and devastating than atomic bombs based on fission. These tests were the cause of a significant pollution near the nuclear test sites.
These fabrication of these thermonuclear weapons require the production of sufficient quantities of tritium. Since natural tritium is extremely rare, this military tritium is obtained by bombarding lithium with neutrons. In addition, tritium from military facilities generates tritium waste, problematic more because of the tritium mobility than its very low radioactive toxicity.
The nuclear fusion of deuterium and tritium will be exploited in reactors based on nuclear fusion. It remains to be proved that such reactors can be designed and built. This is the goal of the research carried out on a worldwide scale with the ITER project.
Other articles on the subject « Main Radioactive Nuclei »
Uranium 238 and 235
A radioactive and strategic element The uranium atom is the heaviest atom present in the natural [...]
Plutonium 239
Plutonium 239: an artificial fissile nucleus, highly sought-after and feared Plutonium, the ninet[...]
Plutonium Properties
A transuranic element with long-lived radiotoxic isotopes Plutonium is a very dense metal, radioa[...]
Radium
The radioactive nucleus that made History Radium is an extremely rare element that was first disc[...]
Carbon-14
A by-product of cosmic rays Carbon-14 (C-14) formation in the atmosphere The nucleus of carbon-14[...]
Potassium-40
A curiosity of Nature and a very long lived beta emitter Potassium 40 is a radioisotope found in [...]
Iodine 131
Radioactive iodine : A dangerous and short lived fission product Iodine 131 is a radioisotope wit[...]
Caesium 137
A legacy of atmospheric nuclear bomb tests and accidents Caesium 137 is a radioactive element wit[...]
Strontium-90
A fission product with properties close to calcium Strontium-90 is with cesium-137 a major radioa[...]
Technetium 99
A pure gamma emitter widely used in nuclear medicine Of all the atoms below uranium in Mendeleyev[...]