Understanding the nature of alpha, beta and gamma rays
Three radiations
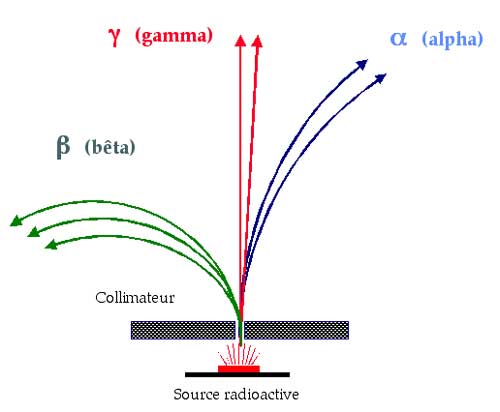
This diagram, copied from an original by Marie Curie, shows the effect a magnetic field can have on different types of radiation. Magnetic fields curve the trajectory of particles carrying an electric charge. Alpha rays, curving to the right, are positively charged, the beta rays curving to the left are negatively charged, and the unaffected gamma rays are electrically neutral. Years later, after 1932, beta particles were observed being curved to the right – this would herald the discovery of the positron, and beta-positive radiation.
© IN2P3
In the years following the discovery of radioactivity, physicists devoted themselves to the study of the properties of the different types of radiations that were emitted by radioactive sources.
The three types that were discovered were classified according to their penetrative ability and electrical charge: and named ‘alpha’, ‘beta’ and ‘gamma’.
Ernest Rutherford identified the nature of alpha and beta radiations. He connected first alpha radiations to helium and later on identified them to helium nuclei after his discovery of the atom nucleus. He interpreted also the emission of beta particles as the emission of electrons discovered a few years before. In 1902, he was able to show that a change in the nature of the atom occurred during the emission of alpha and beta rays : atoms were transmuting.
In 1900, Paul Villard at the Ecole Normale found that gamma rays were simply high-energy photons, and of the same type as X-rays.
Despite being a random phenomenon, radioactivity is governed by a fundamental mathematical law of decay. While in Canada between 1901 and 1903, Ernest Rutherford and his young student Frederick Soddy derived the exponential law of a radioactive decay defined by its ‘half-life’. They proved that the ‘half-life’ was a characteristic property of such a decay, with each element having its own intrinsic half-life.
Physicists were also able to observe the existence of radioactive generations; the polonium and radium Pierre and Marie Cure extracted from pitchblende were direct descendants of the uranium it contained.
Radioactive generations provided valuable evidence for the idea that radioactivity accompanies atomic transmutation, one of the fundamental principles of subatomic physics.
Other articles on the subject « Discoveries »
Marie Curie
Marie Curie Marie Sklodowska-Curie had an exceptional destiny. Born in Poland, she came to gradua[...]
Discovery of the Nucleus
A new vision of the atom In 1911, Rutherford, Marsden and Geiger discovered the dense atomic nucl[...]
Ernest Rutherford
In October 1895, landed in England a 24 years-old young New Zealander. His name was Ernest Ruther[...]
Rutherford’s experiment
The experiment which proved the existence of a nucleus in the atom In 1908, Ernest Rutherford rec[...]
The neutron : Chadwick
A close competition between great physicists … James Chadwick, who discovered the neutron i[...]
Radium and Medicine
A short history: first steps in nuclear medicine … Everyone knows that the discovery of rad[...]
1934 : Artificial Radioactivity
The production at will of radioactive isotopes In the first days of 1934, Frederic and Irene Joli[...]
The Neutrino Hypothesis
The remarkable story of the neutrino The study of radioactive disintegrations had established tha[...]
Fission discovery
A nuclear phenomenon that escaped the hands of physicists In the years spanning 1934 to 1938, Enr[...]
Enrico Fermi
A genial experimenter and theoretician Enrico Fermi (Rome, September 29, 1901 – Chicago, No[...]