A transuranic element with long-lived radiotoxic isotopes

Plutonium isotopes are alpha emmitters
Twice as dense as lead, plutonium has a silver-like aspect in metallic state. Plutonium isotopes are alpha emitters except for plutonium-241. Alpha emitters are not dangerous unless inhaled or ingested. The dead layer of skin is enough to stop them. The penetrating gamma rays that often accompany radioactive decays are missing or have remarkably low energy in the case of plutonium. This explain why this technician can handle this piece of plutonium with simple gloves.
© DR
Plutonium is a very dense metal, radioactive, man-made during the past sixty years as part of its activities involving fissile material. Plutonium isotopes are radioactive. The most abundant isotopes are the 238, 239, 240 and 241. These four radionuclides have radioactive half-lives or periods ranging from 14.1 to 24,000 years. Apart plutonium-241, these isotopes are alpha emitters.
Plutonium-241 decays, by emitting a low-energy beta electron, to Americium-241, whose half-life of 430 years is much longer. Alpha emitter, Americium-241 is much more radiotoxic than its parent. Its maximum activity, reached, after 73 years, is about 3% of the initial activity of plutonium-241.
The fissile property of plutonium-239 and the ability to produce with reactors relatively large amounts of it has led to its use for nuclear weapons and nuclear energy. In reactors, plutonium-239, like uranium-235 captures neutrons and undergoes fission. Plutonium fission provide about one third of the energy produced in conventional reactors. It is not necessary to extract this plutonium which is produced in the nuclear fuelbecause.
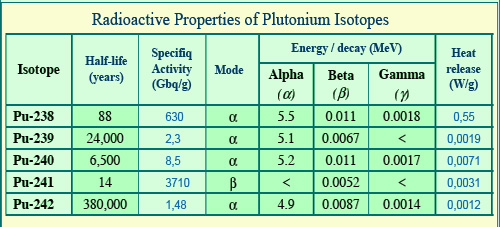
Properties of plutonium isotopes
Plutonium isotopes emit alpha rays except plutonium-241 for which the energy of beta electrons is remarkably low. Most alpha decays are not accompanied by gamma rays, which explains that gamma energy per disintegration is particularly low (1 or 2 keV on average). Periods (half-lives) of 14 and 88 years are relatively short for the isotopes 238 and 241 which are the most active. They are of the order of thousands of years for the other isotopes. The alpha decay of plutonium-238 releases considerable heat.
© IN2P3(Source Encyclopaedia Britannica)
The U.S. has recovered or acquired between 1944 and 1994 approximately 110 tons of plutonium with 100 tons in inventory. About 80% is military grade, mainly plutonium-239, derived from the production reactors at the sites of Hanford and Savannah River.
Atomic weapon tests have dispersed up to 1980s about 10 tons of plutonium into the atmosphere. The level of fallout on the ground is between 10 and 100 picocurie per kilogram (0.37 and 3.7 Bq / kg). Accidents and releases nearby weapon tests facilities have caused locally more important contaminations.
Plutonium occurs most commonly in the form of highly insoluble oxide. It remains in the first centimeters of the ground surface. In water, plutonium is found in the upper layer of sediment to which it strongly adheres. Typically, only one part out of 2000 is dissolved. In soil, chemical or biological processes can make soluble a small fraction of plutonium. But while plutonium may be accumulated in aquatic organisms, there is no accumulation in the food chain.
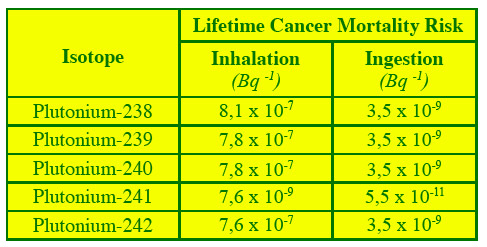
Mortality risk by inhalation and ingestion
The risk of cancer mortality across a lifetime has been calculated for several radionuclides, including plutonium. They are related to a intake by inhalation or ingestion of 1 Bq (Becquerel) of plutonium activity. While risks by ingestion are the most common exposure, the ones by inhalation are greater: 8 out of 10 million people per becquerel against 3.5 out of one billion except in the case of plutonium-241 (beta emitter) for which the risk is lower.
© ANL Fact Sheets
A radiotoxic element
Because of its lack of mobility, medical data do not confirm the reputation of plutonium as the most deadly substance known to man. It is not as immediately harmful as many chemicals. The danger resulting from its alpha radiation does not become effective unless plutonium is present in the human body after inhalation or ingestion.
When plutonium is inhaled, a significant fraction can pass from the lungs into the bloodstream and other organs depending on the solubility of the compound. If plutonium is ingested, the digestive system will only absorbs about 0.05% of it. Very little is absorbed through contact with skin. Plutonium present in the blood is deposited in the liver and skeleton with biological retention periods between respectively 20 and 50 years.
Inhalation of contaminated dust and aerosols is the main risk of cancer resulting from alpha of plutonium, well before the risk in case of ingestion.
Laboratory studies on animals have shown that exposure to high levels of plutonium caused respiratory diseases, cancers and reduced life expectancy. The tissues affected are the lungs, liver, lymph nodes and bones. However, these results have not been corroborated by epidemiological studies on humans exposed to low levels of plutonium
Other articles on the subject « Main Radioactive Nuclei »
Uranium 238 and 235
A radioactive and strategic element The uranium atom is the heaviest atom present in the natural [...]
Plutonium 239
Plutonium 239: an artificial fissile nucleus, highly sought-after and feared Plutonium, the ninet[...]
Radium
The radioactive nucleus that made History Radium is an extremely rare element that was first disc[...]
Carbon-14
A by-product of cosmic rays Carbon-14 (C-14) formation in the atmosphere The nucleus of carbon-14[...]
Potassium-40
A curiosity of Nature and a very long lived beta emitter Potassium 40 is a radioisotope found in [...]
Iodine 131
Radioactive iodine : A dangerous and short lived fission product Iodine 131 is a radioisotope wit[...]
Tritium
A radioactive isotope of hydrogen Tritium is a beta-emitting radioactive isotope of hydrogen. Its[...]
Caesium 137
A legacy of atmospheric nuclear bomb tests and accidents Caesium 137 is a radioactive element wit[...]
Strontium-90
A fission product with properties close to calcium Strontium-90 is with cesium-137 a major radioa[...]
Technetium 99
A pure gamma emitter widely used in nuclear medicine Of all the atoms below uranium in Mendeleyev[...]