Plutonium 239: an artificial fissile nucleus, highly sought-after and feared
Plutonium, the ninety-fourth element in Mendeleyev’s periodic table, is an artificial radioactive nucleus produced in large quantities by reactors when nuclei of uranium 238 capture an extra neutron.

Glenn Seaborg
The American physicist Glenn Seaborg, sitting in front of a Geiger counter when he discovered plutonium. Seaborg was also responsible for improving the safety conditions for those handling radioactive elements, such as the wearing of gloves or masks, after decades of lax security regulations.
© DR
It was first discovered in February 1941 by the American physicist Glenn Seaborg, before America’s entry into the Second World War. The discovery of ‘transuranic’ elements (those with heavier masses than uranium) was a sensational one, and the news was kept secret. Transuranic fissile elements, such as plutonium 239, had a high strategic importance.
Afteri ts discovery plutonium used to be known simply as ‘element 94’. When a name had to be given, inspiration was taken from the outermost planets of our solar system (and the ancient Greek and Roman gods as well). Uranium was named after Uranus, Neptunium (element 93, discovered by Ed. MacMillan) after Neptune. Consequently, element 94 became Plutonium.

The historic B Reactor at Hanford, Washington
Completed in September 1944, the B Reactor was the world’s first large-scale plutonium production reactor. With two other reactors, it produced plutonium for the Trinity device which was tested at Alamogordo, the Nagasaki weapon, and later on Cold War weapons.
© Atomic Archive
Through the framework of the Manhattan Project, the United States built an immense factory near Hanford to extract the plutonium needed for the manufacture of atomic bombs. Among the bombs built at Hanford was ‘Fat Man’ – the atomic bomb that destroyed the city of Nagasaki in 1945.
Plutonium 239 is primarily an alpha emitter, easily transforming into uranium 235, another readily fissile nucleus. The fission of a plutonium nucleus generates an average of 2.91 neutrons, even more than these emitted by uranium 235. Plutonium is therefore an ideal nuclear fuel.
The first nuclear reactors were built to produce plutonium 239 for atomic bombs. These plutonium generators used (and in some cases still use) natural uranium as a fuel: the heavy water Canadian CANDU reactors, for instance, or the first generation French graphite-ga UNGG reactors.
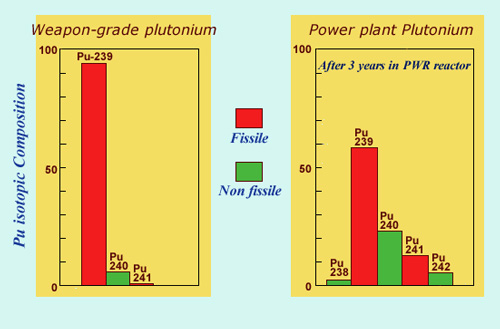
Civilian and military plutonium:
The plutonium used in atomic bombs contains more of the 239 isotope than the plutonium found in civilian reactors. Weapons-grade plutonium is enriched to 93% whereas the plutonium found in PWRs contains around 60%. The presence of 40% of other isotopes makes it inadequate for military uses – in particular, a bomb with a concentration of plutonium 240 above 20%, would be highly unstable.
© IN2P3
Modern reactors can use less enriched uranium to produce a plutonium containing too little plutonium 239 to be used as an explosive but more than enough for it to be a useful fuel.
Plutonium is still the preferred nuclear fuel for the fast neutron surgenerators or breeder reactors. Few of these reactors are operating nowadays but they are generally considered to be those that will power our future.
If it is not burned or used up, plutonium’s long half-life can make it a cumbersome waste product. The half-life of plutonium is of 24,000 years: extremely long on the human time scale but paling into insignificance when compared to the 700 million year half-life of uranium 235. Plutonium therefore either has to be stockpiled in a deep geological repository in such a way that it will pose no threat to future generations, or burned up in nuclear reactors.
Like all heavy atoms, plutonium is a chemical poison. More toxic even than uranium, plutonium is fortunately insoluble in its oxidised state and comparatively immobile. It enters the body through the respiratory more frequently than the digestive system. Inside the body, it can attach itself to bones or organs like the liver or the lungs with a biological half-life of decades.
Glenn Seaborg was the first to take precautions while handling plutonium, using gloves and a mask. The custom between 1941 and 1944 was to handle plutonium practically bare-handed. The quantities were fortunately very small, as plutonium is much more radioactive than uranium. Despite the danger at the time, however, there was no noticeable increase in the mortality rate among the workers and many were still alive forty years later.
TO KNOW MORE ABOUT PLUTONIUM AND ITS USES :
– 2) : Plutonium 239 Formation
– 2) : Plutonium Isotopes
– 3) : Plutonium Properties
Other articles on the subject « Main Radioactive Nuclei »
Uranium 238 and 235
A radioactive and strategic element The uranium atom is the heaviest atom present in the natural [...]
Plutonium Properties
A transuranic element with long-lived radiotoxic isotopes Plutonium is a very dense metal, radioa[...]
Radium
The radioactive nucleus that made History Radium is an extremely rare element that was first disc[...]
Carbon-14
A by-product of cosmic rays Carbon-14 (C-14) formation in the atmosphere The nucleus of carbon-14[...]
Potassium-40
A curiosity of Nature and a very long lived beta emitter Potassium 40 is a radioisotope found in [...]
Iodine 131
Radioactive iodine : A dangerous and short lived fission product Iodine 131 is a radioisotope wit[...]
Tritium
A radioactive isotope of hydrogen Tritium is a beta-emitting radioactive isotope of hydrogen. Its[...]
Caesium 137
A legacy of atmospheric nuclear bomb tests and accidents Caesium 137 is a radioactive element wit[...]
Strontium-90
A fission product with properties close to calcium Strontium-90 is with cesium-137 a major radioa[...]
Technetium 99
A pure gamma emitter widely used in nuclear medicine Of all the atoms below uranium in Mendeleyev[...]