The accident long term legacy
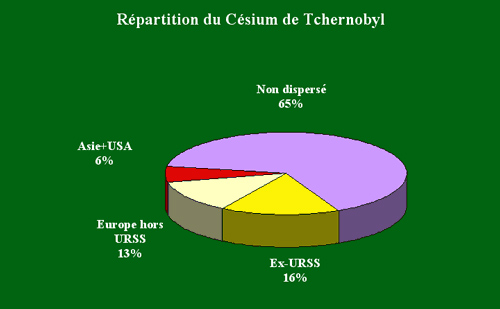
Caesium deposits on the worldwide scale
The 80 000 TBq of caesium-137 released into the environment at the time of the accident in 1986 accounted for one third of the amount that was present in the reactor. From this third about half were deposited in the former USSR and especially near Chernobyl.
© IRSN
More than twenty years after the Chernobyl accident, attention focuses mainly on one particular radioactive nucleus : caesium-137. Iodine-131, dreadful in the weeks following the disaster, has disappeared because of its 8 days radioactive half-life (or period). On the contrary, because of their periods of thirty years, the effects of caesium-137, and to a lesser degree those of one other radioactive element, strontium-90, are still being felt.
The thirty years caesium half-life offers an advantage: it is 1400 times less active than iodine-131. But it carries a drawback: it disappears very slowly.
A French respected intitution, the IRSN, estimates that that 80,000 cesium-137 terabecquerels were released into the environment, i.e. 30 to 40% of the amount present in the the damaged reactor core. The becquerel (Bq) is a very small unit. One uses instead the TBq unit (one million of million of Bq) to represent the Chernobyl releases.
Caesium had disappeared since a long time from the atmosphere after its deposition on the ground. Almost all deposits are found in shallow depth, because migration of this chemical element with minerals is low. It slowly sinks into the ground. More than twelve years after the accident, caesium was concentrated in the top 5 cm of plant litter, contaminating mainly young wood roots and fungi.
Near Chernobyl, the interception of radioactive dust and aerosols by the foliage and then the leaf drop, led to a localized contamination of forest litter on an area of about 40 000 km2. In some areas near the plant, a few centimeters at the surface were removed or covered with uncontaminated soil. This cleanup has reduced the amount of cesium by a factor of 10 to 100.
Caesium contamination, carefully followed by radiation protection agencies, has been the subject of 60 000 measurements since the accident. One observes leopard skin patches of contaminations that follow the pattern of iodine-131 spread in 1986.
Could this radioactive caesium be dispersed again, for instance during fires, and causes a second Chernobyl cloud? The question was asked during the fires that have ravaged some forests in Russia in 2002 and 2010. It should be noted that even large fires cover only a small fraction of the contaminated surface, that a part of this caesium has sunk into the ground and finally that it was short-lived radionuclides such as iodine-131 (and not caesium) that have contributed to the dangerousness of the cloud in 1986. That is why the IRSN has needed ultra sensitive detectors and weeks of observations to detect a small increase in the content of caesium-137, resulting from the 2002 fires.
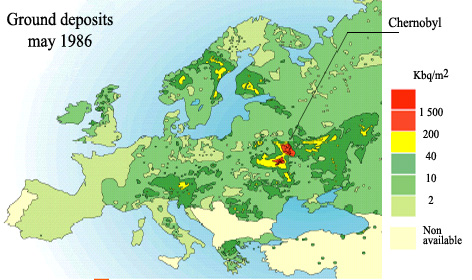
Caesium in Europe after Chernobyl
Measurements have allowed the mapping of the caesium-137 ground activity after the accident. This activity is expressed in kilobecquerels (kBq) per square meter. These deposits added to the residues of the nuclear tests fallouts in the 50’s and 60’S. The map shows contaminations nearly 1000 times higher near Chernobyl than in Western Europe because of the effects of distance. Altogether, Chernobyl deposits represented approximately three times the residual activity of nuclear tests in 1986.
© IRSN
Caesium contamination in France.
The most affected French areas were located in the Vosges mountains and in the Haut Var (Southern Alps) where concentrations of soil deposits have been identified : local topology have played the role of a collector system, accumulating ponctually radioactive elements.
These concentrations, very localized, were among the highest in France. In parts of the some forests, game and fungi showed signs of abnormally high contamination: calculated for one kilogram, the activity has reached 2000 Bq for wild boars and 5000 Bq for some fungi, the normal being a hundred Bq / kg.
Wild boars feed on roots, acorns, and fungi located in the first centimeters of forest soils. The mycelium, part of the perennial fungi grows at these depths and this vast underground network concentrates minerals, mainly potassium. It traps also caesium, chemical analogue of potassium, as it traps, also pesticides.
The risks associated with those concentrations should be however reduced to their just proportions. The IRSN has calculated that a woodman eating only wild game and fungi would be subject to an annual dose of 1 mSv, 40% of the dose due to natural radioactivity in France.
NEXT: Chernobyl : Iodine-131
Other articles on the subject « Chernobyl accident »
Chernobyl Circumstances
An explosion followed by a graphite fire In 1986 the Ukrainian Chernobyl plant had 4 RBMK reactor[...]
Chernobyl Liquidators
A heavy toll paid by firefighters and liquidators: The magnitude of the disaster took the Soviet [...]
Radioactive Releases
Radioactive releases and contaminations at Chernobyl The explosion of the reactor led to the disp[...]
Chernobyl Plume
A deep mark in the french collective unconscious « The Chernobyl cloud has stopped at the french [...]
Chernobyl Exclusion Zone
A no man’s land turned into a natural reserve ? On April 27th, 1986, one day after the expl[...]
Chernobyl Health Effects
Chernobyl: A toll still impossible to quantify It is difficult to reach an approached assessment [...]
Chernobyl Iodine 131
A dangerous radioelement during the first weeks Iodine-131 is the most feared fission product whe[...]
Chernobyl Today
The Chernobyl Site Over 30 Years Later On December 15, 2000, the Ukrainian government, in accorda[...]