1896 : The first clue of a new phenomenon

Wilhelm Konrad Roentgen
Wilhelm Konrad Roentgen, a German physicist, announced the discovery of X-rays on the 28th of December 1895. The X-ray scan of his wife’s hand was published in newspapers all around the world and caused an instant sensation. His work was rewarded with a Nobel Prize for Physics in 1901.
© Musée Curie
On the 28th of December 1895, Wilhelm Conrad Roentgen announced the discovery of X Rays. The scan of his wife’s hand was published in newspapers all around the world and caused an instant sensation.
As of mid-January 1896, doctors and physicists in all Western countries were using the X-ray apparatus described by Roentgen to perform scans of their own. The Parisian physicist Oudin and the doctor B. Barthelemy made the first French X-ray scan, the results of which were presented to the Academie des Sciences by Henri Poincare on the 20th of January. At the presentation, Poincare suggested to Henri Becquerel that he investigate whether a relationship existed between phosphorescence and X-ray emission.
Henri Becquerel belonged to what could almost be called a dynasty of curious thinkers and scientists that were working at the time. He worked in a laboratory at the Natural History Museum in Paris and devoted much of his time to exploring the relationship between phosphorescence and fluorescence.
Becquerel then decided to see whether or not X-ray emission was linked to phosphorescence. On an overcast day in March 1896, unable to expose a phosphorescent uranium salt to the sun, he placed the uranium on top of a fresh sheet of photographic paper at the back of a drawer. Some days later he discovered that the salt had spontaneously emitted a penetrative ray capable of leaving an impression on the photographic plate.
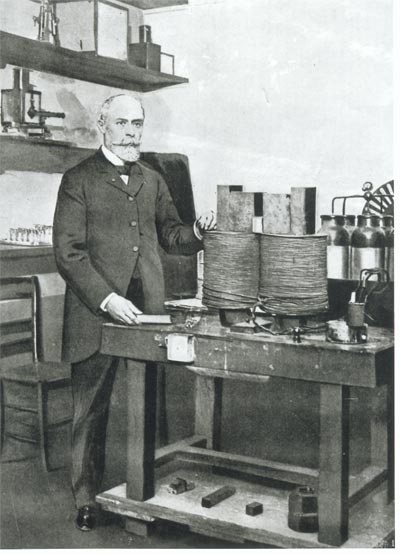
Henri Becquerel in his laboratory.
Henri Becquerel (1852-1908), a graduate of the Polytechnic School, belonged to what could almost be called a dynasty of curious thinkers and scientists that were working at the time. He worked in a laboratory at the Natural History Museum in Paris and devoted much of his time to exploring the relationship between phosphorescence and fluorescence.
© ACJC
Becquerel quickly established that a variety of uranium salts, whether phosphorescent or not, have the same property; proving that the radiation is an intrinsic characteristic of uranium.
He referred to the emitted rays as ‘uranic rays’, and was able to show that they could electrify the air. As the intensity did not diminish over time, Becquerel started asking himself: Where does uranium get the energy that it emits so consistently?
THE MALTESE CROSS : An unexpected clew of a new phenomenon
We know today that « uranium rays » which acted on the Becquerel plate were not alpha particles coming from uranium. They were stopped by the paper envelope. The acting rays were instead beta and gamma rays coming from the first uranium descendants.
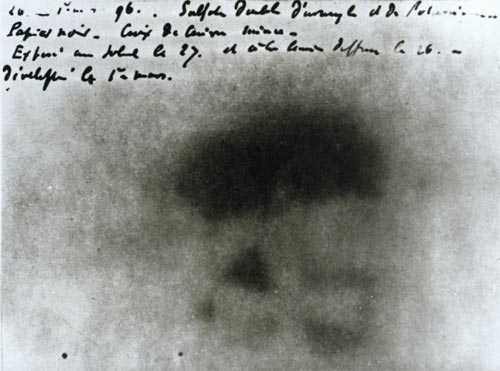
Image of the Maltese Cross.
One of Henri Becquerel photographic plates – developed on 1 March 1896 – shows the image of a Maltese cross. The copper cross was interposed on February 26, 1896 between the photographic plate wrapped in paper and a thin glass slide supporting salts of uranium. All ended up in a dark drawer. The appearance of the cross is due to the fact that the copper has absorbed the radiation emitted from uranium salts.
© ACJC
Henri Becquerel had used a flask containing salts of uranium, a rare product, however available to the National Museum of Natural History. Given the radioactive period of uranium-238 and its descendants, the flask should contain the first filiation elements, that is to say, thorium-234, protactinium-234 and uranium-234.
Uranium-238 (main isotope of uranium) decays by emitting alpha particles which are quickly absorbed by the material. As Henri Becquerel’s photographic plates were covered of thick paper, the alpha particles could not be the cause of the plate marks.
Among the descendant of uranium-238, protactinium-234 decays by emitting highly energetic beta rays. Five to six millimeters of glass are required to completely absorb these electrons. Thus they have contributed to draw the Maltese Cross interposed by Henri Becquerel between the plate wrapped in paper and a thin glass plate of 0.1 mm withstanding uranium salts.
Remain different gamma rays emitted by the descendants of uranium. These gamma rays have contributed to act on photographic plates. They were attenuated by the interposition of glass slides more or less thick, and sheets of aluminum or copper. Despite their low intensity and the values of corresponding attenuation coefficients, they provided the « continued success » of the experiences of pioneers.
Other articles on the subject « Discoveries »
Three radiations
Understanding the nature of alpha, beta and gamma rays In the years following the discovery of ra[...]
Discovery of the Nucleus
A new vision of the atom In 1911, Rutherford, Marsden and Geiger discovered the dense atomic nucl[...]
Ernest Rutherford
In October 1895, landed in England a 24 years-old young New Zealander. His name was Ernest Ruther[...]
Rutherford’s experiment
The experiment which proved the existence of a nucleus in the atom In 1908, Ernest Rutherford rec[...]
The neutron : Chadwick
A close competition between great physicists … James Chadwick, who discovered the neutron i[...]
Radium and Medicine
A short history: first steps in nuclear medicine … Everyone knows that the discovery of rad[...]
1934 : Artificial Radioactivity
The production at will of radioactive isotopes In the first days of 1934, Frederic and Irene Joli[...]
The Neutrino Hypothesis
The remarkable story of the neutrino The study of radioactive disintegrations had established tha[...]
Fission discovery
A nuclear phenomenon that escaped the hands of physicists In the years spanning 1934 to 1938, Enr[...]
Enrico Fermi
A genial experimenter and theoretician Enrico Fermi (Rome, September 29, 1901 – Chicago, No[...]