Slowing fast neutrons without capturing them
The presence of a moderator; is essential for operating reactors whose fuel is poor or slightly enriched in fissile elements. The purpose of this moderator medium is to slow the neutrons to promote fission.
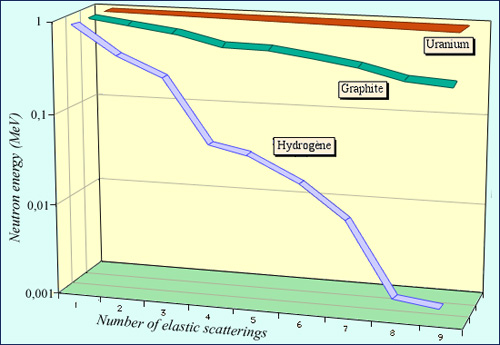
Comparison of three neutron slowdowns
The figure shows how the neutron energy decreases due to successive collisions on nuclei present in the environment where they operate. About ten collisions on the hydrogen protons are sufficient to divide by 1000 the energy of a neutron. The slowdown is a bit slower in deuterium. It is much slower with a lightweight nucleus such as carbon (which has 12 nucleons) and especially uranium that contains 238 nucleons. The initial energy of the neutron was chosen 1 MeV.
© IN2P3
Fission neutrons are slowed down by successive collisions on the moderator nuclei. The aim is to avoid as least as possible neutron capture during this series of collisions: the moderator must be « transparent. » The slowdown should be fast to avoid captures by other nuclei. To thermalize a 2 MeV neutron it takes on average 26 collisions in an hydrogenated environment, 31 in deuterium, 120 in carbon and 2202 in uranium.
The rapidity of the slowdown with hydrogen is due to the fact that hydrogen is composed of protons, which have almost the same mass as neutrons. Hydrogen is the fastest medium for slowdown, but has the disadvantage of capturing some neutrons to form deuterium, the heavy isotope of hydrogen.
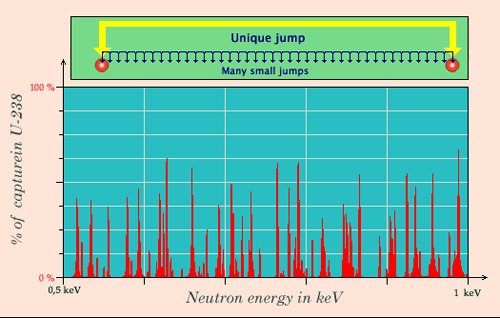
Avoiding « resonant captures »
In a reactor core, neutrons below 10 keV enter in the region of uranium-238 resonances. The figure gives a partial picture of this domain where capture probabilities are important for certain energies, and almost 0 elsewhere. In order to get slow neutrons to operate the reactor, neutrons must remain a short time in this resonance area. With a water moderator, a collision on a proton of the hydrogen allows a neutron to make leaps above these turbulences by halving its energy on average. Without a moderator, the neutron loses its energy by small jumps and has a high probability of being captured by one of the resonances.
© IN2P3 (Source JANIS)
The deuterium, in the form of heavy water, is a better moderator. It is a little less « fast », but being already composed of a proton and a neutron, it captures fewer neutrons than hydrogen. It is an ideal retarder, whose handicap is to be expensive (one finds one deuterium atom for 6500 atoms of hydrogen in Nature). Moderated with heavy water, a reactor is able to produce energy with natural uranium. For instance, the CANDU Canadian and Indian reactors use heavy water as moderator
Graphite is also a very effective moderator. Carbon is a very stable nucleus reluctant to accept a new neutron. The first nuclear reactor designed by Enrico Fermi in 1942, which functioned during the Second World War with natural uranium was « moderated » using graphite. Graphite is abundant, but as it must be cleansed of impurities, it is expensive.
The first plants using natural uranium required moderators such as deuterium (heavy water) and graphite to operate. When enriched uranium became available as fuel, ordinary water became suitable as a moderator and a coolant. Most existing reactors usually operate now with pressurized or boiling water.
NEXT : Slow and Fast neutrons
Other articles on the subject « Neutronic Radiations »
Neutron Capture
Capture competes with fission and generates radioactivity The neutron is a special elementary par[...]
Neutron Balance (fission)
What happens to fission neutrons? Within a reactor the objective is to maintain the chain reactio[...]
Slow and fast neutrons
Fission depends on the energy of the neutrons Nuclear fission can occur when a nucleus is rendere[...]
Slow neutrons
Slow neutrons favour fission In nuclear reactors slow neutrons are neutrons slowed down after a s[...]
Fast neutrons
Fast neutrons for surgeneration and breeders Before they are slowed down by a large number of nuc[...]