The experiment which proved the existence of a nucleus in the atom
In 1908, Ernest Rutherford received the Nobel Prize for identification of alpha particles with helium. During his Nobel Prize speech, he specified that these atoms of helium were doubly ionized. This result causeed a sensation because it was the result of ultimate measurements made before his trip to Sweden.
Rutherford did not stop there, he continued his researches on the properties of radioactive radiations. In the list of experiments to do after his return from Stockholm, he registered the diffusion of alpha particles, that is to say how they are deflected when travelling through matter.
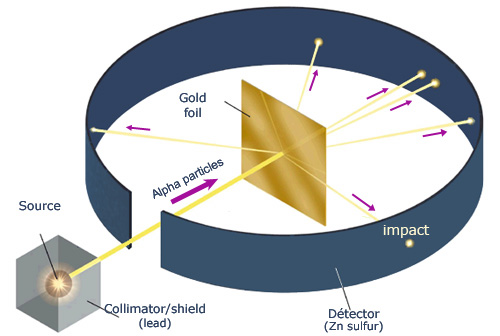
Principle of Rutherford’s experiment
By bombarding a very thin gold foil with alpha particles, Hans Geiger and Ernest Marsden, both students of Rutherford, observed that a small fraction (1 in 8000) of these particles were deflected at large angle as if it bounced off a heavy obstacle. The impacts were observed as scintillations in the dark, under the microscope, on a screen of zinc sulphide. Rutherford concluded that the atom contained a heavy heart, with positive electric charge, able to push away the alpha
© DR
In 1903, Philip Lenard, bombarding atoms with cathode rays had noticed that they passed through the atoms as if they could find almost nothing on their trajectory. He summarized his observations by saying that at atomic scale « the solid matter is transparent » and noticed that « the space occupied by one cubic meter of solid platinum is as empty as the space between the stars and the earth » .
Observing that high speed alpha particles were deflected by a thin sheet of mica, Rutherford calculated the electric field inside the mica and deduced that it should have been very powerful. But he thought he should be able to detect and count deflected alpha particles. With his assistant Hans Geiger, Rutherford developed a method to do so. The result of their observations confirmed the existence of strong electric fields. Nevertheless an enigma remains: a few alpha particles were deflected.
Rutherford asked a young assistant Ernest Marsden to see if alpha particles were subjected to a high deflection, and even bounced back, when they went through a thin gold foil. That was the case! It was in darkness and with the naked eye, that Rutherford, Geiger and Marsden counted the scintillations due to the impacts of alpha particles on a screen of zinc sulphide.
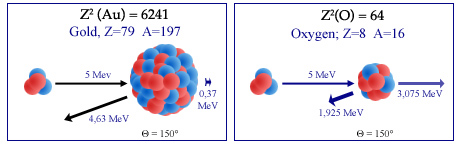
Alpha backscattering on nucleus
Rutherford observed the backward bounce of some alpha particles as projectiles sent on atoms of a thin gold foil. He interpreted this rebound as the « backscatter » of a light nucleus (alpha particle) on the heavy nucleus of a gold atom. Because of the mass ratio (4 versus 197), the alpha particle bouncing off (on the left figure) at 150 °, loses only a small portion of its energy transfered to the gold nucleus – which allow to emerge from the gold foil. On the contrary, in the case of a collision (on the right) with a lighter nucleus like oxygen (A = 16), the alpha loses most of its energy, propelling the of oxygen forward.
© IN2P3
Rutherford pointed out in a phrase made famous, that it was as “It was as if you fired a 15 inch shell at a piece of tissue paper and it came back and hit you » (It was years later that he made his famous comparison ! ). Rutherford studied the phenomenon for a year and found the explanation: the atom positive charge is in a solid and compact nucleus. This nucleus concentrates almost the entire mass of the atom but occupies only a hundred millionth of a millionth of its volume. The atom is almost one hundred percent empty.
Related topic : The neutron : Chadwick
Other articles on the subject « Discoveries »
Marie Curie
Marie Curie Marie Sklodowska-Curie had an exceptional destiny. Born in Poland, she came to gradua[...]
Three radiations
Understanding the nature of alpha, beta and gamma rays In the years following the discovery of ra[...]
Discovery of the Nucleus
A new vision of the atom In 1911, Rutherford, Marsden and Geiger discovered the dense atomic nucl[...]
Ernest Rutherford
In October 1895, landed in England a 24 years-old young New Zealander. His name was Ernest Ruther[...]
The neutron : Chadwick
A close competition between great physicists … James Chadwick, who discovered the neutron i[...]
Radium and Medicine
A short history: first steps in nuclear medicine … Everyone knows that the discovery of rad[...]
1934 : Artificial Radioactivity
The production at will of radioactive isotopes In the first days of 1934, Frederic and Irene Joli[...]
The Neutrino Hypothesis
The remarkable story of the neutrino The study of radioactive disintegrations had established tha[...]
Fission discovery
A nuclear phenomenon that escaped the hands of physicists In the years spanning 1934 to 1938, Enr[...]
Enrico Fermi
A genial experimenter and theoretician Enrico Fermi (Rome, September 29, 1901 – Chicago, No[...]