Leaving a wake in the sea of atoms…
An electrical charge gives a particle the ability to act over large distances, allowing it to remove electrons from atoms in the material it passes through. This phenomenon is known as ‘ionization’, and the neutral atoms that have lost electrons have become ‘ions’. The greater the electrical charge on a particle, the greater its ionizing capability.
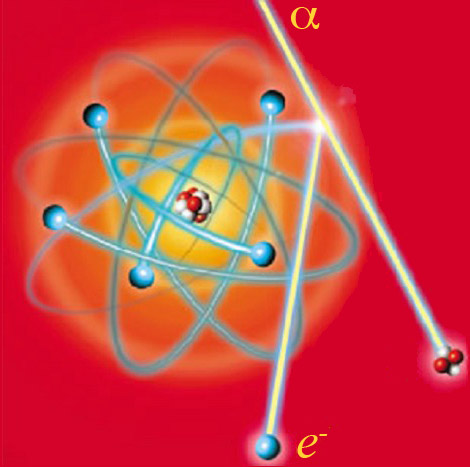
Ionization of an atom
A charged particle (here an alpha particle) withenough energy expels an electron from an individual atom lying on its path. The atom with the missing electron becomes an ion, with its electron structure disturbed. The energy transferred by the alpha particle is eventually released in the form of electromagnetic radiation or heat. The alpha particle can also liberate distant electrons thanks to its electric charge. Most particles ionize hundreds of thousands, if not million of atoms before stopping.
© DR
An alpha or beta particle has an energy some hundreds of thousands of times larger than the few electronvolts (eV) needed to ionize most atoms. It would take some 30 eV to remove an electron from a gaseous atom, and far less to remove one from a crystalline structure. The silicium crystals used in certain detectors (and microchips), for instance, only need 3 eV for an electron to be liberated. An alpha particle with an energy of 3 MeV (millions of electronvolts), therefore, can either ionize some 100,000 gaseous atoms or close to a million silicium crystals atoms.
The energy loss due to ionization is proportional both to the particle’s mass and to the square of its charge. It also changes dramatically with speed: when the particle is slower, it spends more time in the atom and has a higher chance of interacting with it while it passes through. As a result, ionization becomes particularly intense near the end of the trajectory, when the particles have lost most of their energy. This property is frequently used in therapy; the irradiation of cancerous tumours relies on curving trajectories so that the radioactive particles target the malignant cells.
Liberating many electrons uses a great deal of energy, and the particles finally come to a halt. The greater the ionization capability, the shorter the distance these particles can cover. This is especially true for the alpha particles, where the length of the trajectory depends on their initial energy. Beta particles have winding tracks that nevertheless cannot surpass a certain maximum length characteristic of the initial energy. From a protection point of view, a layer of armour that is thicker than this maximum length should achieve the required levels of safety.
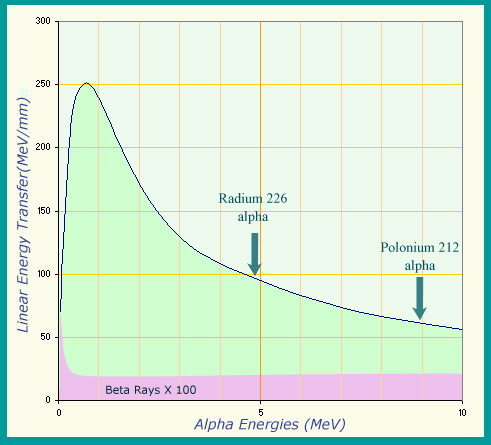
Highly ionizing alpha particles
The ionizing power of a charged particle is measured by its ‘linear transfer of energy’ : the energy it loses per unit length in the material it goes through. Alpha particles are particularly ionizing, as can be seen by the variation in energy transfer in water as a function of their energy. While the energy transferred by an alpha-induced radioactive decay never goes above 9 million electronvolts (9 MeV), their ionizing power equates to between 60 and 250 MeV per millimetre during the slow down. Beta particles are far less ionizing: the values had to be multiplied by 100 to appear on the same graph.
© IN2P3
The electrons and the ions they leave behind generally recombine soon after the ionizing particle has disappeared. The energy lost is quickly dissipated as heat, enough heat to start producing bubbles in a liquid on the verge of boiling. In beer, for instance, these bubbles are caused by particles charged by cosmic radiation. Ionization has multiple effects: the breaking of molecular bonds, the creation of free radicals, catalyzing chemical reactions, generating structural flaws in crystalline atoms, etc… In certain transparent plastics, the atoms return to equilibrium by emitting light.
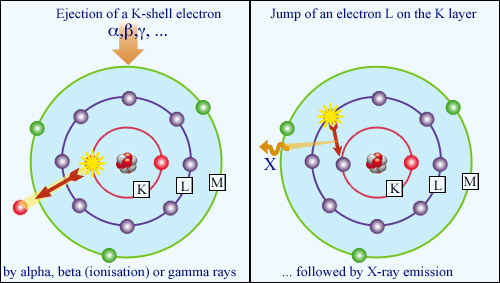
Atomic restructuring : X-ray fluorescence:
When the expelled electron (whether freed by ionization or gamma-induced Compton effect) belongs to the inner K layer of the atom, the atom reacts by emitting a characteristic and highly penetrative X-ray. This is the phenomenon of X-ray fluorescence, which has a wide range of applications.
© IN2P3
When the expelled electron (whether liberated by ionization or the Compton effect caused by gamma radiation) comes from the inner core of the atom, the atom reacts by emitting a characteristic and highly penetrative X-ray. This is the phenomenon of X-ray fluorescence, which has a wide range of application.
Other articles on the subject « Radiations effects in matter »
Alpha Rays in Matter
An atomic bulldozer, strongly ionizing along a very short path Alpha particles are simultaneously[...]
Beta Rays in Matter
Light electrons : a chaotic journey through matter Beta electrons and positrons have equal and op[...]
Bremsstrahlung
A relativistic phenomenon that applies to electrons and positrons… The phenomenon of bremss[...]
Neutral Particle Effects
Energy transfer by proxy… Neutral particles that are of interest in the field of radioactiv[...]
Cherenkov Effect
When an electron goes faster than light in air and water … The Cherenkov effect occurs when[...]
Cross Section
Cross section or the interaction probability of a particle Cross section is the name given by phy[...]
Gamma Rays in Matter
Gamma can be attenuated but never fully stopped The neutral gamma rays leave very different effec[...]
Photoelectric Effect
The most effective mechanism of photon absorption The photoelectric effect is the phenomenon that[...]
Compton Effect
Photons as projectiles and electrons as targets The Compton effect is the name given by physicist[...]
Macroscopic Effects
Effects on inert or organic matter The ionisation of atoms surrounding the trajectory of an alpha[...]