Mechanisms of atomic nuclei formation
Most of the nuclei of atoms that make up our daily life were formed in the furnace of stars, and for others during violent stellar cataclysms.
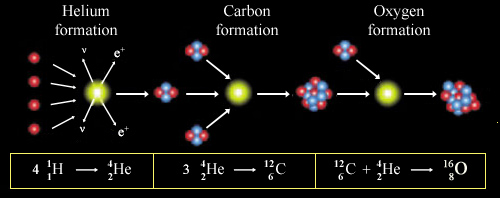
Synthesis of light nuclei
In the sun, helium is produced (simplifying) by the fusion of 4 hydrogen nuclei. It’s the energy released by the fusion reactions that mainly provides us warmth and light. The stars also synthesize other elements. By fusion reactions of light nuclei, heavier and heavier elements are formed up to iron. Then the fusion stops, because the formation of heavier nuclei require external inputs of energy.
© IN2P3
Stars are formed from a cloud of material composed primarily of hydrogen. This cloud is contracting as a result of attraction due to gravity. The gravitational collapse of the cloud (called proto-Sun) heats the medium until the first reactions can « turn on », those which will convert hydrogen into helium. Then, other light nuclei are formed among them carbon and oxygen.
This is what the sun does since 4.5 billion years and will still do during about the same time. These fusion reactions release energy, which radiates some form of light and heat. The pressure of this radiation prevents the star from contracting further.
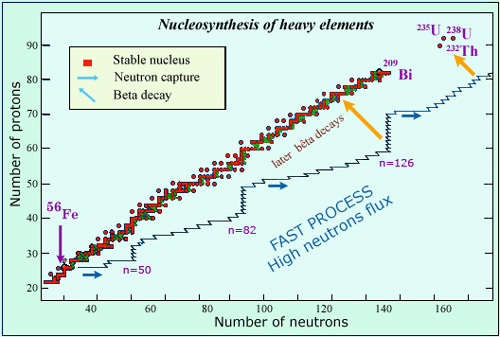
From Iron to uranium
The central core of very massive stars is rich in iron, the most stable nucleus in nature created from previous nuclear fusions. How to explain then the presence of heavier nuclei in the universe, some very heavy like radioactive thorium and uranium ? It is believed that during supernovae explosion an extraordinarily intense neutron flux is produced. Bombarded by such a flux, the iron nuclei grow very rapidly through successive neutron captures. These nuclei would follow on the nuclear chart a path that takes them away from the line of stability (in red). Later on, beta decays transform them into the heavy elements of today.
© IN2P3/NEPAL
When the hydrogen fuel is exhausted, nothing precludes a new gravitational collapse. The temperature will rise even more until the new fusion reactions of nuclei begin until the production of iron (iron-56) is reached. Iron together with nickel-62 which is next to it, are the nuclei with the greatest nuclear stability. The ultimate fusion reactions that lead to iron can only occur within the central core of stars much larger than the sun.
Beyond iron, nature uses a different known mechanism to synthesize the heaviest nuclei (gold silver, lead, uranium). This mechanism occurs at the last stage of the life of very massive stars, which ends with an explosion. The star becomes very bright: a supernova.
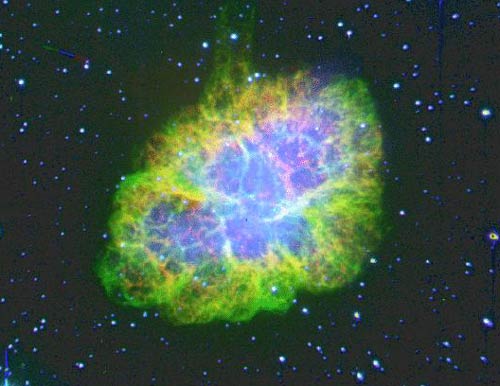
The dispersion of the stellar material
The Crab nebula is the remnant of a supernova explosion that occurred 6000 years ago in our galaxy and that has been observed on Earth in 1054 by Chinese astronomers. The supernova phenomenon is due to the explosion of a big star at the end of its life. During such explosions are produced by a rapid succession of neutron captures, elements heavier than iron, such as platinum, gold or uranium. These elements are dispersed by the explosion in the galactic space.
© PALOMAR
The explosion seeded galactic space of stardust, containing heavy nuclei. Much later, this dust will condense with the interstellar gas to form new solar systems and in particular planets like our familiar Earth.
Other articles on the subject « Radioactive nuclei »
Map of Nuclei
Map of stable and unstable nuclei The progress made in our understanding of the subatomic world o[...]
Stability Valley
Beta decay : nuclei getting slimmer to achieve stability The nucleus mass is related to its inter[...]
Nuclear Forces
Three nuclear forces and their hierarchy Three types of force act alongside each other inside a n[...]
Mecanisms of Radioactivity
The way radioactivity works and its origins The impressive range of half-lives that exist, extend[...]
α decay : tunnel effect
Particle and wave: an effect of quantum mechanics The great age of uranium and thorium nuclei tha[...]
Alphas with gammas
Gamma rarely accompanies alpha decays It is surprising that one can hold with hands a sample of p[...]
β decay : weak forces
The forces which allow a nucleus to emit beta electrons Beta decay (β) and electronic capture cha[...]
Weak Forces
A special and fascinating fundamental interaction A third force is at work in the nucleus next to[...]
The Nucleosynthesis
Primordial and stellar nucleosynthesis The Universe hasn’t always existed, It was born a li[...]