The ancient dream of the alchemists…
Examples of transmutation
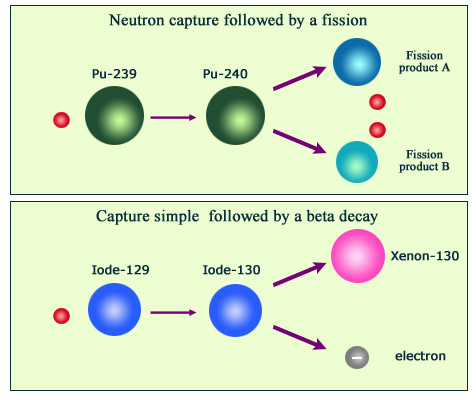
The figure shows two examples of nuclear transmutations sparked off by neutrons in nuclear reactors. Above, a neutron capture causes a nucleus of plutonium 239 to split and generate two smaller nuclei, known as fission products. Below, a second neutron is captured by an iodine 129 nucleus that transforms into iodine 130. Iodine 130 then emits a beta particle, causing it to transmute into a stable nucleus of xenon 130, a noble gas atom quite different also from Iodine.
© IN2P3
The alchemists of the Middle Ages and the Renaissance worked in vain to transmute lead into gold. Modern nuclear physicists are the heir of their legacy, substituting Bunsen burners and beakers for reactors and accelerators in an attempt to transform the basic building blocks of matter. While the lucrative transformations dreamt of by alchemists are as yet impossible, nuclear physicists regularly create nuclei of atoms from other nuclei: transmutation. In this, as in so much else, science is following in the footsteps of Nature, where such nuclear changes have been occurring spontaneously since the beginning of the Universe.
Any change made to the nucleus of an atom instantly affects the structure of the atom itself. As soon as the nucleus has emitted an alpha particle, say, or an electron or positron, the delicate balance of electric charges that exists in the atom is broken, with the nucleus. In order to regain stability, the atom is forced to either release some of the orbiting electrons, or attract in more from outside.
Once these exchanges have finished, the atom’s basic chemical nature has changed. This phenomenon was first noticed by Ernest Rutherford (an omnipresent figure in the early history of radioactivity!), in 1900. Rutherford’s experiment was to observe the emanation of a radioactive gas, later known as radon, in the disintegration of metallic radium.
In 1901, along with his pupil Frederick Soddy (1877-1956), he was able to show that both alpha- and beta- emissions are indicative of a fundamental change in the nature of the nuclei and atoms involved. As well as providing evidence for the controversial ‘solar system’ model of the atom by proving the existence of a nucleus, he was thus able to conclusively disprove one of science longest-held dogmas: the indivisibility of matter. The age-old belief that ‘the atom could not be split‘. Soddy later recalled that when, in the sheer exultation of the moment, he triumphantly claimed to have witnessed ‘transmutation’, Rutherford warned him: « For Christ’s sake, Soddy, don’t call it transmutation. They’ll have our heads off as alchemists. »
Today, such ‘transmutation’ is basic currency for all physicists, and can take place on relatively large scales. At the heart of nuclear reactors, where uranium is transformed into plutonium, a one Gigawatt reactor can produce 200 kilograms a year; admittedly, a modest result compared to the amount of energy involved. While there is nothing to theoretically prevent the eventual transformation of lead into gold, a metal detector and a goldpan are still your best bets
Other articles on the subject « Alpha Beta Gamma rays »
Alpha (α) radioactivity
How heavy nuclei lose weight … by emitting alpha particles Alpha (α) radiation was first ob[...]
Beta (β) radioactivity
How Nature corrects an excess of protons or neutrons Beta (β) radioactivity was first observed in[...]
Beta spectrum
Beta electrons do not have a unique characteristic energy Three particles, the recoil nucleus, th[...]
Electron Capture
A minor mode … competing with positron emission Electron capture is a comparatively minor d[...]
Positron
The positive electron, the first ambassador of antimatter The positron was discovered in 1933 by [...]
The electron-neutrino
An electron that would have lost its electric charge The easiest way to conceive a neutrino is to[...]
Muons
An heavy electron abundant in cosmic rays Muons were first observed in 1936 by the Americans phys[...]
Radioactivity Gamma (γ)
How nuclei get rid of excess energy It was in 1900 that the French physicist Paul Villard first f[...]
Nuclear Desexcitations
Gamma are the light emitted by atomic nuclei A french proverb used to say « all roads go to Rome.[...]
Internal Conversion
When a gamma expells an atomic electron and is absorbed Internal conversion is a nucleus desexcit[...]