Nuclear variants of a given atom …
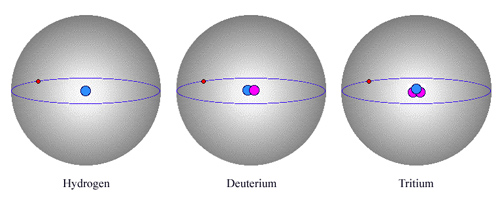
The three isotopes of hydrogen
The hydrogen atom has two isotopes: deuterium and tritium. The nucleus of hydrogen consists of one solitary proton, deuterium has one neutron and a proton and tritium contains one proton and two neutrons. The atom electrical charge are the same, and the chemical properties are identical. There are also differences, however. Deuterium is twice as heavy as hydrogen (and tritium is three times heavier), and is stable, though much rarer than hydrogen. Tritium, on the other hand, is unstable and radioactive
© IN2P3
Free of all electric charge and present only in the nucleus, neutrons play a minimal role in determining the atom behaviour and its chemical properties. As a result, two different atoms of the same chemical element can have unequal numbers of neutrons; such atoms are known as ‘isotopes‘.
A good example is that of hydrogen and its two isotopes, deuterium and tritium. As all three atoms have nuclei containing only one proton, they have one negatively charged electron in orbit so as to maintain electrical neutrality. The chemical properties of the three atoms, as well as the types of light they emit and absorb, are also identical. A nucleus of hydrogen, however, is half as heavy as one of deuterium, and has only one third the mass of a tritium nucleus, since the proton and neutron masses are practically equal.
Another helpful example involves the isotopes of the element carbon. A normal carbon atom contains 6 electrons orbiting a nucleus where 6 protons and 6 neutrons are tightly packed together. The negatively charged surrounding electrons are in no way affected by the addition or removal of neutrons (which do nothing to change the total electrical charge), and its chemical properties as well. The nucleus may get heavier or lighter, but the atom stays as an atom of carbon.
Different versions of the same chemical element, ‘isotopes‘, can mainly be distinguished by the properties of their nuclei. While having no effect at all on an atom’s chemical properties or electrical charge, a change in the number of neutrons can have a substantial influence on the behaviour of the nucleus. Adding or removing neutrons can shift the nucleus’s delicate equilibrium, and has the ability to change its stability. The isotope of carbon with 6 protons and 8 neutrons (known as carbon 14), for instance, commonly found in the Earth’s atmosphere, has an unstable nucleus and is consequently radioactive. The presence of carbon 14 is used to date remnants of by-gone ages.
Natural uranium is made up of two isotopes : uranium 235 and uranium 238. The former present only at the level of 0.7 % is used in nuclear reactors because it is fissile, while uranium-238 is not.
Virtually all radioactive isotopes have disappeared from our daily lives ; the only exceptions are those whose half-lives are very long (as is the case for uranium), those that are constantly being produced by natural reactions (such as carbon 14 and all descendants of uranium), and all those that humanity is able to produce in its reactors and accelerators.
Other articles on the subject « The atomic nucleus »
The proton
The charged constituent of the nucleus The nucleus of the hydrogen atom consists of one solitary [...]
The Neutron
The neutral partner of the proton The neutron is, along with the proton, one of the two constitue[...]
Nucleus Energy Levels
Analogies with the atomic energy levels … At first glance, nuclei seem to be very different[...]
Quarks and leptons
The fundamental constituents of matter On the most elementary scale, the world around us is made [...]
Quarks and Gluons
How quarks interact within nuclei … To describe radioactivity and nuclear reactions such as[...]
Matter and Antimatter
Nature loves symmetry. However, one can not dream of a matter less symmetrical than the matter wh[...]