Chronicle of the life of a fission fragment …
The fission of a nucleus into two fragments is by far the most common. The two fragments are extremely radioactive and unstable when just formed. Each of them will seek to reach stability through a series of transformations. The return to stability can take very different times. The journey resembles to that of a train that has to pass through several stations before reaching the terminus. The terminus is a nucleus situated at the bottom of the stability valley.
Les us consider as an example the fission of an uranium-235 nucleus into two fragments of 140 and 94 nucleons.
Production of two fragments with 140 and 94 nucleons.
The heaviest fragment is a radioactive isotope of xenon which has 4 more neutrons (86) than the nearest stable xenon isotope. This heavy fragment will evolve keeping its 140 nucleons, through the successive emissions of four beta electrons to reach a stable cerium-140 nucleus. This cerium-140 has four neutrons less (82) and 4 more protons (58) that the initial xenon-140 fragment.
Coming back to the train comparison, the travel time to the first station is the time for the xenon-140 to decay into cesium-140 : the half-life of this first stage is 13.6 seconds, extremely short. The duration of the cesium-140 decay – the second stage – with a 63 seconds half-life is also very short. The duration of the third stage, the baryum-140 decay, is much longer with a 12.7 days half-life. Finally, the duration of the final stage is given by 1.67 days of the lanthanum-140 decay. In total, the travel time to reach the cerium-140 terminus will be around fifteen days, of the order of the longest 12.7 days half-life.
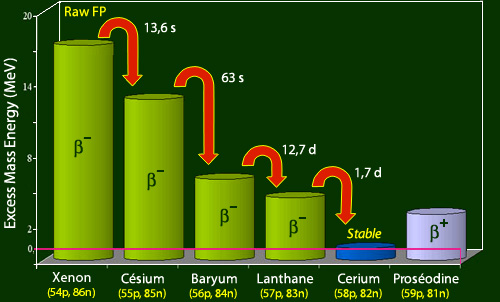
Evolution of the 140 nucleons fragment
Within the family of 140 nucleons, the lightest and most stable is a cerium isotope, cerium-140 (shown in blue). The diagram shows the mass (i.e. internal) energy difference of the nuclei of this family with respect to the mass of this cerium-140 nucleus, the nuclei being classified here according to their number of protons. The raw fission fragment, a nucleus of xenon-140, is on the left. It loses its mass energy excess, jumping 4 stairs steps, to arrive to the stable cerium-140. Each jump is accompanied by a beta electron emission.
© IN2P3
The lighter strontium-94 fragment follows for its part a similar evolution, to reach a stable zirconium-94 nucleus.
This scenario is general. Raw fission fragments far away from stability, reach stability through the two means made available to them by Nature: expulsion of neutrons or beta decays. The expulsion of neutrons is rare because it is costly in energy. It may occurs only early in the cascade of transformations, and become energetically forbidden at later stages. Expelled neutrons are produced some time after the fission itself and its primary neutrons. Although marginal in numbers, these delayed neutrons play an important role in the control of the chain reaction in reactors.
It is the beta decay which mainly ensure the return to stabilities. The duration of this return depends mainly on the longest half-life in the decay cascade. In the xenon-140 and strontium-94 example, transformations into stable fission products are fast. This is not always the case. The arrival at the bottom of the stability valley terminus can take a long time. A fragment of 137 nucleons will take 30 years to see the final stage of cesium-137 decaying into barium-137. A 99 nucleons fragment of technetium-99 will take 100,000 years to turn into stable ruthenium-99.
Nuclear fuels stay usually 3 to 4 years in the core of reactors. During this long stay, most of the fission fragments would have time, like the xenon-140 fragment, to lose their radioactivity. But others fail to reach stability. It is these fission products, slow to complete the final stage towards stability, which are responsible for much of the radioactivity of the spent nuclear fuels of reactors.
NEXT : Fission products
NEXT : Shorl Lived Fission Products
NEXT : Long Lived Fission Products
Other articles on the subject « Nuclear Fission »
200 millions electronvolts !
A huge amount of energy at the atomic scale The fission of a uranium or plutonium nucleus liberat[...]
Chain Reaction
From one single fission to fissions on a massive scale Nuclear fission emits a lot of energy on t[...]
Fissile nuclei
Uranium 235 and 233, plutonium 239 The few fissile nuclei found in nature belong to heavy atoms, [...]
Fission products
The ashes of nuclear fission Fission products are the remains of a heavy uranium or plutonium nuc[...]
Plutonium-239 formation
Transforming a fertile nucleus into a fissile nucleus Uranium-238 accounts for more than 95% of t[...]
Short-lived Fission Products
Most fission products vanish rather rapidly The vast majority of the radioactive fission products[...]
Long-lived Fission Products
A handful of tough but little radioactive isotopes … The more a nucleus is long-lived, the [...]
Actinides
A class of very heavy radioactive nuclei Alongside the fission products found in the core of nucl[...]
Plutonium Isotopes
Plutonium-239 but also 238, 240, 241, 242 … Plutonium isotopes are produced by neutron capt[...]
Minor Actinides
Neptunium, americium and curium Minor actinides constitute a very small minority of high activity[...]