Most fission products vanish rather rapidly
The vast majority of the radioactive fission products are short-lived or medium-lived. For radioactive waste management, a radionuclide is said short-lived when its period is less than 5 years and medium-lived when it is between 5 and 100 years. Such timescales seem very long, but they are actually short compared to the half-life (period) of some fission products or actinides, which are counted in thousands of years. A period of 30 years, such as that of cesium-137, appears rather long when it comes to handle the radioactive contamination resulting from a nuclear accident. Instead, a physicist, who often has to deal with radioactive nuclei living less than a second, would sea periods of a few hours or days as « longs ».
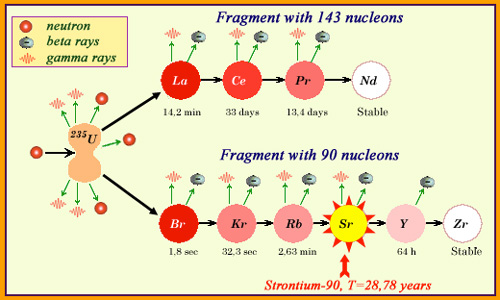
Double cascade …
The products found in reactor spent fuels are no longer the fresh fragments of an Uranium-235 fission. Here, a heavy fragment of 143 nucleons passes through 3 intermediate nuclei, whose period ranges from 14 minutes to 33 days, to reach the stable nucleus terminal of the chain (neodymium-143). The light fragment of 90 nucleons evolves rapidly at the beginning, but the cascade remains stranded with the fourth element, the strontium-90 whose period is around 30 years. It will take about 10 times that long for the fragment radioactivity to disappear. Strontium-90 is one of the major radioactive fission products found in reactors.
© IN2P3
These radioactive life times are to be compared with the residence time of the nuclear fuel in the reactors which is usually three years. When the period is very short – for example a few days – much of the radioactive nuclei have reached stability when the fuel is discharged from the core. Will be present at a given time only freshly produced such nuclei . On the contrary, if the half-life is of a few years, the radioactive nuclei will accumulate because they do not have had time generally to disappear.
Examples of very short-lived fission products are xenon-135 (period 8 hours) and iodine-131 (period of 8 days). The accumulation of xenon-135 after a reactor shutdown becomes a poison for fission reactions and prevents its immediate restart. Iodine-131 is one of the fresh fission products that were released during the Chernobyl accident. This highly mobile and volatile element was the main radioactive hazard immediately after the accident, since it is estimated that around 10% of fission products present in the reactor have been dispersed in the atmosphere.
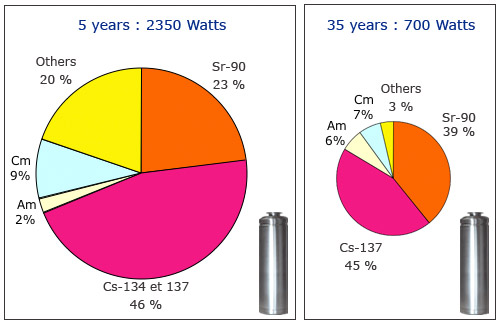
Heat from a container of vitrified waste
Containers of vitrified waste from the nuclear industry are too recent to have had time to age. They still emit excessive heat caused mainly by the decay of fission products contained therein. At the age of 5 years, the short-lived fission products contribute about 90% of the heat release and the minor actinides (americium and curium) for only 11%. At age 35 the heat of 2350 watts fell to 700 watts, two products of fission – Cesium-137 and strontium-90 – alone emit 84% of this heat.
© CNE
Fission products such as cesium-137 (30-year half-life or period) and strontium-90 (28-year period) have « intermediate » life times. They live long, but not extremely long. They are troublesome, because if they are much less active than iodine-131 for instance, they disappear slowly. They represent the main source of contaminations due to the Chernobyl accident and former tests of atomic bombs decades after these events.
Fission products are responsible for virtually all the radioactivity of spent fuel just removed from a reactor core: 99.5% of beta and gamma activity. This activity is very high, estimated to be about 2.5 million curies ( 7 times 10 to the power 16 becquerels) per ton of nuclear spent fuel.
Before handling spent fuel assemblies, it is necessary for radioprotection to store these assemblies inside pools by letting their radioactivity decrease a few years : first in a pool close to the reactor and, in the case of a reprocessing, inside the interim storage pool of the reproceessing plant (la Hague in France) where they are transfered. The storage pools are also designed to evacuate the heat generated by the radioactive decays.
Other articles on the subject « Nuclear Fission »
200 millions electronvolts !
A huge amount of energy at the atomic scale The fission of a uranium or plutonium nucleus liberat[...]
Chain Reaction
From one single fission to fissions on a massive scale Nuclear fission emits a lot of energy on t[...]
Fissile nuclei
Uranium 235 and 233, plutonium 239 The few fissile nuclei found in nature belong to heavy atoms, [...]
Fission fragment
Chronicle of the life of a fission fragment … The fission of a nucleus into two fragments i[...]
Fission products
The ashes of nuclear fission Fission products are the remains of a heavy uranium or plutonium nuc[...]
Plutonium-239 formation
Transforming a fertile nucleus into a fissile nucleus Uranium-238 accounts for more than 95% of t[...]
Long-lived Fission Products
A handful of tough but little radioactive isotopes … The more a nucleus is long-lived, the [...]
Actinides
A class of very heavy radioactive nuclei Alongside the fission products found in the core of nucl[...]
Plutonium Isotopes
Plutonium-239 but also 238, 240, 241, 242 … Plutonium isotopes are produced by neutron capt[...]
Minor Actinides
Neptunium, americium and curium Minor actinides constitute a very small minority of high activity[...]