An energy concentrate based on uranium-235
Fissile nuclei like uranium 235 or plutonium 239 are extraordinarily concentrated stockpiles of energy, with one gram of these substances capable of liberating as much energy as a ton of petrol!
The nuclear fuel placed in the reactor core contains fissile isotopes, these nuclei that give off tremendous amounts of energy when they are split. The quality of the nuclear fuel available – in particular the abundance and the nature of the fissile atoms – is a determining factor in the design of the reactor and how it will be operated.

Nuclear Fuel assemblies
Nuclear fuel assembly of pressurized water reactor (PWR) photographed under water. Cladding coating the uranium oxide pellets stacked inside long « rods » can be seen. A group of rods or pencils are held together to form a fuel assembly measuring approximately 4, 50 m long and weighing half a ton.
© CEA
The most common nuclear fuels are those derived from uranium, as uranium 235 is the only readily fissile isotope in nature. Even though it makes up less than 0.7% of all the naturally-occurring uranium ore, it is always possible to enrich natural uranium: a process whereby the percentage of uranium 235 is increased. The long and complex techniques used to enrich raw uranium for civilian purposes have been developed in industry since the 1960’s
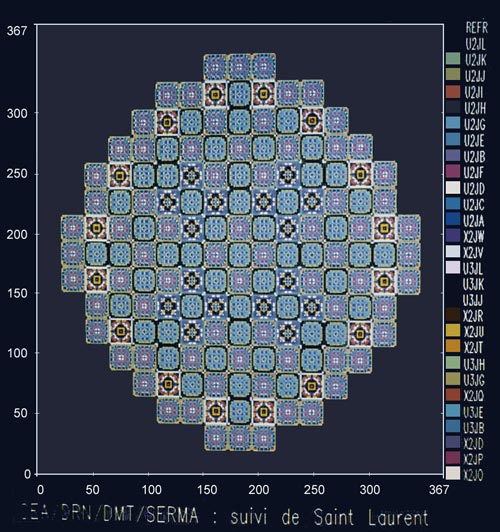
Layout of a reactor’s core
Layout of the core of a pressurized water reactor, made during studies of plutonium recycling. The diagram shows the MOX fuel assemblies containing plutonium (in blue) among the majority of those containing usual enriched uranium (UOX).
© CEA
While generating electricity conventional reactors also produce significant quantities of plutonium, another fissile element which can be recycled into the fuel. This process increases the total amount of fissile material available, allowing for an increased production of energy.
Using plutonium made in this way to enrich the nuclear fuel is an excellent way of recycling radioactive waste. MOX fuel (mixed oxide fuel), a combination of uranium and plutonium, has been regularly used, notably in France and Japan.
The fuel is inserted into the reactor in the form of uranium oxide (UO2) pellets, piled up in zirconium cans to be arranged in ‘pencil’-shaped rods, which are then assembled in bundles. The main advantage of using uranium oxide is that it can stand high temperatures more easily.
As the fuel produces its energy, the number of fissile nuclei gradually decreases. A few fission products can absorb a large number of neutrons and ‘poison’ the way reactors work. The best-known example is xenon 134, which can prevent the restart a reactor by several hours.
In order to keep the power output, despite the loss of reactivity with time, a variety of compensating procedures can be used until, after about 3 or 4 years, the fuel has become too poor to be of any use. For this reason, about a third of the reactor fuel is replaced every year.
Within today reactors, fissile materials are introduced through solid fuels, such as the pellets of uranium oxides of PWR reactors. The option of introducing these fissile materials into a liquid medium, such as molten salts, is possible. The alternative was tested with convincing results in the 1960s in the US, at the Oak Ridge National Laboratory. It is being considered now as one of the option of fourth-generation reactors. The use of liquid fuels would in principle offer advantages. For example, it would not be necessary to shut down the reactors to reload fuel, the production of long-lived waste such as minor actinides would be reduced, an in-line reprocessing could remove a large part of its radioactivity from the reactor core.
Articles on the subject « Nuclear Fuel »
Cycle Front End
From the extraction of uranium to the fuel fabrication The front-end of the nuclear cycle is the [...]
Isotopic Separation
The access key to the fuel of modern nuclear power plants … and to atomic weapons The uranium fue[...]
Ultracentrifugation
A separation process economical in energy, but proliferating The principle of centrifuges has lon[...]
Gaseous Diffusion
The first enrichment process, a large consumer of electricity The gaseous diffusion process was t[...]
PWR fuel assemblies
Fuel assemblies in the core of PWR Reactors The core of a pressurized water reactor (PWR) is cont[...]
Reactor fuel layout
Fuel assemblies in the core of nuclear reactors The nuclear fuel assemblies, that vary from one r[...]
Plutonium Use
A fissile element produced in nuclear reactors In February 1941 the American physicist Glenn Seab[...]
Pu : Fuel, bomb or Waste ?
Plutonium dark and bright sides … Sensitive material, like the roman god Janus, plutonium h[...]
MOX fuels
Introducing plutonium in nuclear fuels For energy production, plutonum is gold ! One gram of plut[...]
Thorium Fuels
Uranium 233: a fissile nucleus made from thorium Thorium is more abundant than uranium in the Ear[...]