A handful of tough but little radioactive isotopes …
The more a nucleus is long-lived, the less radioactive it is. This obvious statement is often overlooked, but this is the case of long-lived fission products whose lifetimes are of the order of hundreds of thousands or a million years. For waste management, a radioelement is said long-lived if its radioactive half-life (or period) is beyond 30 years. These fission products, which represent about 6% of the total, are troublesome mainly because they are slow to disappear. It should be noted that the drawback of extremely long lives is offset by very low radioactivity.
Long-lived fission products
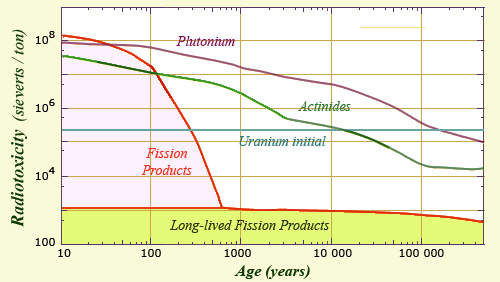
The inventory of the radiotoxicity of spent fuel shows that the contribution of fission products decreases « relatively » quickly until about 600 years. At that time scale, fission products like cesium-137 and strontium-90 whose periods are 30 years have given way to a handful of radionuclides having very long periods. The activities varying inversely to periods, these fission products contribute little to the radioactivity and toxicity. Their radioactive toxicity is less than 0.5% of that of the initial uranium ore and less than one hundred millionth of the initial radiotoxicity of the spent fuel.
© Source : Clefs CEA
After an hundred years of age, the activity of fission products in the nuclear spent fuel from drops with the disappearance of medium-lived elements. After 500 years, fission products represents only a thousandth of that of plutonium and minor actinides. This activity is more than fifty-thousandth of what it was at the time of discharge from the reactor.
After a dramatic decrease during the first 500 years, the radioactivity of fission products reached a plateau that will last for hundreds of thousands years, that is to say the time needed for long-lived elements to disappear in their turn. During this time, about as long as the Quaternary period, the activity of fission products will be only a few thousandths or hundredths of those actinides, whose activity has also decreased considerably.
This residual radioactivity of fission products is due to a handful of long-lived isotopes. Key members of this group are technetium-99, cesium-135, iodine-129, palladium-107 and zirconium-93. Zirconium and palladium are chemically not very mobile, which leaves cesium, iodine and technetium as potential troublemakers. These elements are more mobile than actinides. Iodine-129 and cesium-135 are known for their proneness to leave the chemical structures in which we try to keep them and their tendency to spread in the environment.
Technetium-99 is the main nuclide of this trio, being the most abundant and the most active with the shortest period of 215,000 years. It is possible to destroy it by transmutation, the neutron capture transforming technetium-99 within a few minutes into a stable nucleus. This nuclear reaction, which requires to back to a nuclear reactor, however, is difficult to achieve with a good yield.
Iodine-129 is the most toxic if swallowed, but its very long period of 15.7 million years makes it very little active. By comparison, it is 240 million times less active than its short-lived and dangerous companion, iodine-131 which was the cause of thyroid cancer around Chernobyl. The effects of cesium-135 are the same as those of cesium-137 (the main legacy of radioactivity at Chernobyl 20 years later), but with a period of 2.3 million years, its activity is 77 000 times lower.
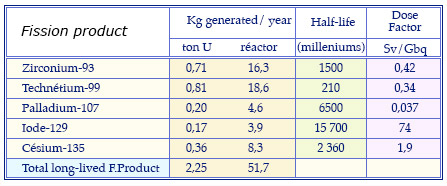
Characteristics of the main long-lived fission products
Masses of the fission products generated annually in long-lived fuel of a conventional pressurized water reactor that has undergone a standard combustion of 33 gigawatt-days. These quantities are given for one ton and 23 tons of uranium fuel (annual consumption). The table recalls the periods of these radioactive elements and their dose factor, estimate of the potential toxicity if swallowed.
© Source clefs CEA N46 and CNE 2003 report
The risks presented by these few longlived radioelements are minimal. There are plans to transmute, as stated above, elements such as technetium.
Let us imagine that, by a hundred years, our descendants decide to get rid of long lived fission products by sending them beyond the solar system, and they fire a rocket loaded with 11 tons of technetium, the technetium production during 10 years by the 60 French reactors. Let us imagine also that unfortunately, the rocket with these 11 tons ended up in a mountain lake a cubic kilometer volume, where all the technetium is dissolved. What would the impact of this disaster? The calculation shows that one should drink about one cubic meter of water from this lake to be exposed to a dose equal to one year of exposure to natural radioactivity.
Other articles on the subject « Nuclear Fission »
200 millions electronvolts !
A huge amount of energy at the atomic scale The fission of a uranium or plutonium nucleus liberat[...]
Chain Reaction
From one single fission to fissions on a massive scale Nuclear fission emits a lot of energy on t[...]
Fissile nuclei
Uranium 235 and 233, plutonium 239 The few fissile nuclei found in nature belong to heavy atoms, [...]
Fission fragment
Chronicle of the life of a fission fragment … The fission of a nucleus into two fragments i[...]
Fission products
The ashes of nuclear fission Fission products are the remains of a heavy uranium or plutonium nuc[...]
Plutonium-239 formation
Transforming a fertile nucleus into a fissile nucleus Uranium-238 accounts for more than 95% of t[...]
Short-lived Fission Products
Most fission products vanish rather rapidly The vast majority of the radioactive fission products[...]
Actinides
A class of very heavy radioactive nuclei Alongside the fission products found in the core of nucl[...]
Plutonium Isotopes
Plutonium-239 but also 238, 240, 241, 242 … Plutonium isotopes are produced by neutron capt[...]
Minor Actinides
Neptunium, americium and curium Minor actinides constitute a very small minority of high activity[...]