The ashes of nuclear fission
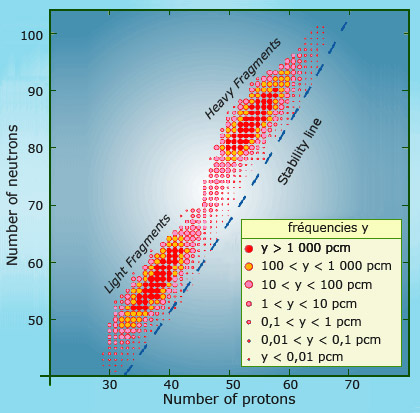
Two unequal fragments
The diagram above shows the location of the fission products of uranium 235 on the map of nuclei. As one can see, the nucleus splits into two unequal fragments. There are two distinct groups of nuclei, with the more abundant fission products coloured in red (the abbreviation phm indicates one part per hundred million). The nuclei produced by the fission of uranium also inherit uranium neutron surplus, which leads to their instability and radioactivity.
© NUCLEUS
Fission products are the remains of a heavy uranium or plutonium nucleus which splits apart after capturing a passing neutron. As the ‘ashes’ of a nuclear reaction, they provide the bulk of the radioactivity found inside the spent fuel in nuclear reactors.
An uranium or plutonium nucleus generally splits into two unequal pieces – a veritable Laurel and Hardy double act with one light nucleus containing between 80 and 110 nucleons and another heavier one made up of between 130 and 155 nucleons.
A nucleus of uranium 235 (made up of 143 neutrons and 92 protons) contains 61% neutrons, whereas the stability level for heavy or light nuclei is under 57%. As a result, the two new fragments are highly radioactive at the moment of their creation, as the excess number of neutrons they inherit from the parent nucleus renders them unstable. These two new nuclei gradually regain stability by undergoing a series of beta decays, which transform the excess neutrons into protons.
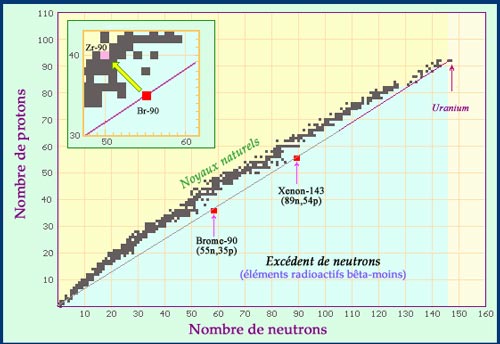
Fission fragments have an excess of neutrons
Newly born fission products inherit the neutron-proton ratio of their parent nucleus. On the map of nuclei, the fragments of uranium fission are located on the straight line which links the uranium-238 nucleus to the origin. In the example above, one can see that Br-90 and Xe-143 are both in the blue area of nuclei with an excess of neutrons, able of emitting beta-negative radiations. The two fragments will finally join the black area of stable (or quasi-stable) nuclei through a chain of beta decays. The insert shows the path undertaken by the fragment of bromine-90 as it decays into a stable nucleus of zirconium-90.
© IN2P3
This ‘cascade‘ of nuclear transformations starts off very quickly, but the time taken to reach stability varies with the decaying fragment. A nucleus with 140 nucleons will stabilise in a few days, whereas one with 137 nucleons will take 30 years and a nucleus with 99 nucleons will remain radioactive for 210 millennia.
Around two thirds of all fission products stabilise in the time they are stockpiled inside the core of a nuclear reactor. When the spent fuel is moved after a couple of years, the radioactive fuel therefore still contains a substantial proportion of unstable nuclei. The most radioactive of these, with the correspondingly shortest half-lives, will disappear fairly quickly. It is paradoxically the least radioactive nuclei that are the most problematic, as they will continue to be a low key nuisance for many generations from now.
In the two hundred years immediately following a fission reaction, the fission products are much more radioactive than the actinides (the heavy elements which are also found in spent fuel and later in nuclear waste). After these 200 years, however, most of the fission products will have stabilised and it is the actinides that become the main hazard.
The term ‘fission products’ applies to a very wide range of chemicals. Many intereact with oxygen and can be found as solid oxides in the spent fuel. The most mobile fission products are gases, such as iodine-131 that decays rapidly. Very mobile fission products such as krypton-85 are noble gases, not forming molecules : their radioactivity is the less toxic.
Other articles on the subject « Nuclear Fission »
200 millions electronvolts !
A huge amount of energy at the atomic scale The fission of a uranium or plutonium nucleus liberat[...]
Chain Reaction
From one single fission to fissions on a massive scale Nuclear fission emits a lot of energy on t[...]
Fissile nuclei
Uranium 235 and 233, plutonium 239 The few fissile nuclei found in nature belong to heavy atoms, [...]
Fission fragment
Chronicle of the life of a fission fragment … The fission of a nucleus into two fragments i[...]
Plutonium-239 formation
Transforming a fertile nucleus into a fissile nucleus Uranium-238 accounts for more than 95% of t[...]
Short-lived Fission Products
Most fission products vanish rather rapidly The vast majority of the radioactive fission products[...]
Long-lived Fission Products
A handful of tough but little radioactive isotopes … The more a nucleus is long-lived, the [...]
Actinides
A class of very heavy radioactive nuclei Alongside the fission products found in the core of nucl[...]
Plutonium Isotopes
Plutonium-239 but also 238, 240, 241, 242 … Plutonium isotopes are produced by neutron capt[...]
Minor Actinides
Neptunium, americium and curium Minor actinides constitute a very small minority of high activity[...]